Barrow Neurodegeneration Scientist Earns High-Risk, High-Reward Grants
The National Institutes of Health (NIH) recently awarded two R21 grants to Barrow Neurological Institute scientist Rita Sattler, PhD, to support innovative research into neurodegenerative diseases.
One study will investigate, for the first time, whether a specific protein can trigger cellular changes linked to amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). The other will take a unique approach to examine disease progression in dementia with Lewy bodies versus Parkinson’s disease dementia.
R21 grants encourage exploratory and developmental research by funding the early and conceptual stages of potentially groundbreaking projects. These high-risk, high-reward grants cap funding at two years.
Dr. Sattler, a professor in the Department of Translational Neuroscience at Barrow, and her laboratory gathered the preliminary data for both grants with the support of donor-directed funds from Barrow Neurological Foundation.
TDP-43 and Neurodegeneration: What We Know
One of Dr. Sattler’s projects builds upon existing research implicating TDP-43 in neurodegenerative diseases, in which nerve cells deteriorate or die.
Most cells in the human body contain the TDP-43 protein. It normally resides in the control center of the cell, known as the nucleus, and plays a role in many activities that are necessary for cells to function and survive.
More than 15 years ago, researchers found TDP-43 mainly located outside of the nucleus in FTD and ALS. The protein had instead accumulated into toxic clumps within the cytoplasm, or the semifluid substance surrounding the nucleus.
Follow-up studies suggested that this abnormal event, called TDP-43 proteinopathy, significantly contributes to neurodegeneration.
“It’s not just the buildup that is contributing to cell death but also the fact that we’re losing the protein in the nucleus,” Dr. Sattler said. “That’s what we call a loss of function, while at the same time we have a gain of function due to the accumulation in the cytoplasm. So it’s really a double whammy.”
TDP-43 Misplacement: Unmasking the Chaperones
While scientists have made strides in understanding the consequences of this protein’s misplacement and buildup, the trigger for this process has remained elusive. The R21 grant will provide $345,567 over two years to support Dr. Sattler, her team, and their collaborators as they study their proposed mechanism. This mechanism involves molecular changes in the nucleus known as A-I RNA editing. The researchers hypothesize that a protein known as ADAR2 can trigger these changes.
RNA converts genetic information from DNA into proteins through a process called transcription. Cells can make changes to this information through a naturally occurring process known as RNA editing. Preliminary data from the Sattler Laboratory supports the idea that increased A-I RNA editing transforms existing RNA molecules into chaperones that can bind to and guide TDP-43 outside of the nucleus.
Dr. Sattler and her team aim to help reveal the molecular identity of these chaperones, in hopes of pinpointing an early event in the disease process and thus a potential target for new drug treatments.
“If we want to therapeutically intervene, we always want to go as far upstream in the disease process as possible,” Dr. Sattler explained. “Downstream, there may be 10 things that go wrong because of one single event. The higher up in the pathways we go, the higher our chances are that we’re really making a significant contribution.”
If we want to therapeutically intervene, we always want to go as far upstream in the disease process as possible. … The higher up in the pathways we go, the higher our chances are that we’re really making a significant contribution.
Rita Sattler, PhD, Professor, Department of Translational Neuroscience
Dr. Sattler and her staff stumbled upon this mechanism somewhat by accident. Much of the Sattler Laboratory’s research focuses on what’s happening at the molecular level in the genetic mutation C9orf72, the leading known genetic cause of ALS and FTD. They began to home in on the ADAR2 protein because of its role in RNA editing.
Whenever the researchers saw dysfunction in ADAR2, they also noted dysfunction in TDP-43. Further exploration suggested this wasn’t a coincidence; ADAR2 can apparently regulate TDP-43’s exit from the nucleus.
“It was really serendipity, totally unexpected,” Dr. Sattler said. “Then we just followed up and found this really cool data, and now we’re really diving into this.”
Dr. Sattler credited much of this work to a former graduate student in her lab, whose thesis project formed the basis of the R21 grant. Stephen Moore, PhD, now works as a postdoctoral fellow at the University of California San Diego in the Department of Cellular and Molecular Medicine. Gene Yao, PhD, a professor in that department, is a collaborator on the R21 grant. Dr. Sattler has also teamed up with Kendall Van Keuren-Jensen, PhD, at the Translational Genomics Research Institute (TGen).
Simplifying the System to Mimic Cellular Changes
The Sattler Laboratory will use mammalian cell lines, or collections of cells originating from one cell, to test their hypothesis. Although not neurons, these neuron-like cells are easy to genetically manipulate.
“Obviously we’re interested in how this works in a neuronal setup because we’re interested in the function of these proteins in the brain, but we’re taking one step back in this approach to simplify the system,” Dr. Sattler said.
The team will artificially increase or decrease the activity of ADAR2 in these cell lines to study how that protein’s activity affects the function and location of TDP-43.
TGen will analyze the transcripts, or gene readouts, of these cells and evaluate their overall wellbeing. The researchers can then compare changes at the transcription level in these neuron-like cells to brain autopsy tissue.
“We’ll be able to see whether there are overlapping dysregulations and loss or gain of gene expression, and we can determine if that parallels anything that we mimic in our simple model system,” Dr. Sattler said.
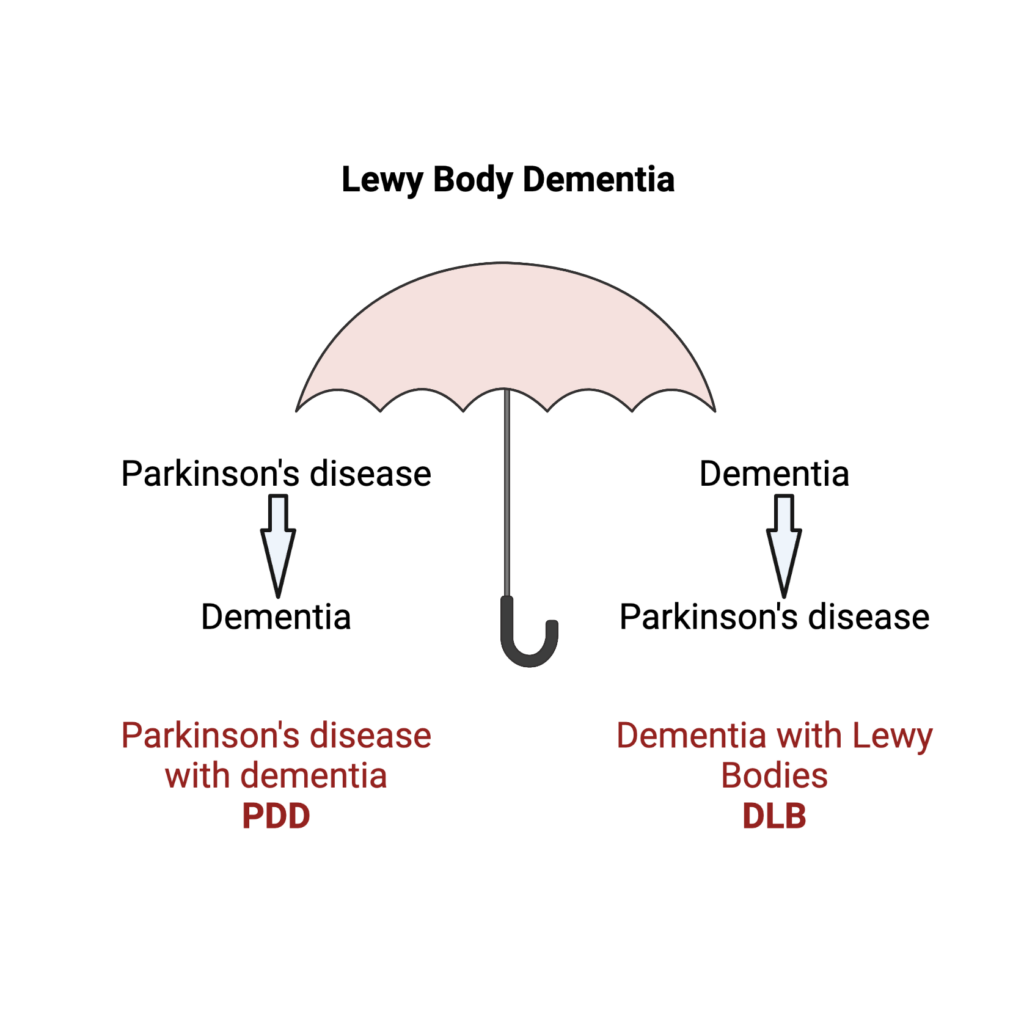
Differentiating Lewy Body Dementia Types
The NIH has also awarded Drs. Sattler and Van Keuren-Jensen an R21 grant of $427,960 for a project focused on Lewy body dementia (LBD). The LBD spectrum consists of two disorders: dementia with Lewy bodies and Parkinson’s disease dementia. While the two share clinical symptoms and disease characteristics, such as the presence of abnormal protein clumps known as Lewy bodies, they differ in terms of progression in the temporal lobe of the brain.
Individuals who have dementia with Lewy bodies show signs of cognitive decline before developing movement-related symptoms. People with Parkinson’s disease dementia, however, develop motor symptoms first and memory changes second.
Drs. Sattler and Van Keuren-Jensen, both principal investigators on the grant, are collaborating with Thomas Beach, MD, PhD, and Geidy Serrano, PhD, to examine the temporal progression of these two dementias.
Validating the ‘Brain in the Dish’
For this work, the Sattler Laboratory will use nerve cells that have been reprogrammed from blood or skin cells donated by living patients. These are known as patient-derived human induced pluripotent stem cells (iPSCs).
These cells will come from people who have also volunteered to donate their brains to scientific studies after death. This will allow the researchers to compare iPSC-neurons to postmortem tissue from the same individuals.
We are hoping it will give us a first indication on how reflective our ‘brain-in-the-dish’ model system is of what’s really happening in the individual.
Rita Sattler, PhD
“So these are matching samples, and that is still a very unique opportunity,” Dr. Sattler explained. “It will be a small sample size, but we are hoping it will give us a first indication on how reflective our ‘brain-in-the-dish’ model system is of what’s really happening in the individual.”
Dr. Sattler explained that a challenge with neurodegeneration research is the inability to access deteriorating brain tissue from a living patient.
“With brain tumor research, tumors are going to be removed,” Dr. Sattler said. “You can get access to the tumor and study it. That doesn’t work for us, so we have to find the best model system possible.”
This project will—for the first time—investigate the transcriptome profiles, cellular characteristics, and disease mechanisms in these two dementias.
From Curiosity to the Clinic?
Dr. Sattler said that existing data didn’t drive this study but rather curiosity.
“We’ve obviously been interested in different types of dementia, and Lewy body dementia is a very interesting dementia type because it combines a motor dysfunction,” she said. “It was a unique opportunity where we thought this could be a really good way to see how accurately we can measure and mimic progression of a disease using those iPSCs.”
Ultimately, Dr. Sattler hopes this project could improve the lives of patients with Lewy body dementia by uncovering potential drug targets for treatment and biomarkers for diagnosis.
“A patient would show up in the clinic, and you could do a quick test to objectively diagnose the patient with Parkinson’s disease dementia or dementia with Lewy bodies based on the biomarkers that we develop,” Dr. Sattler said. “That accurate diagnosis determines the type of care patients receive.”