News & Articles
Home / About Barrow Neurological Institute / News & Articles

Read More
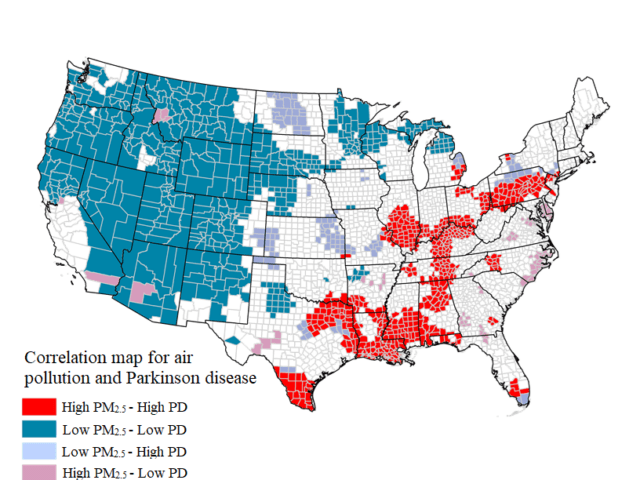
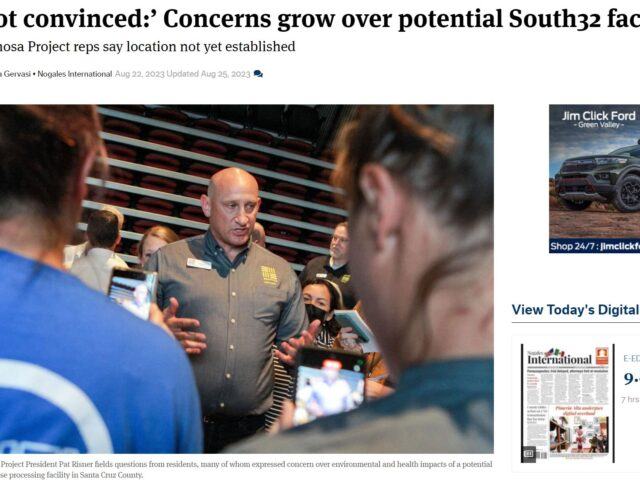
Arizona Mining Project Sparks Concerns for Air and Water
A study led by Dr. Brad Racette, a leading researcher on clinical…
Read More