
An Experimental Granuloma Model in Rat Spinal Cord
T. Erhan Cosan, MD
Stephen W. Coons, MD*
Curtis A. Dickman, MD†
Sacit G×ulec, MD‡
Esref Tel, MD
Departments of Neurosurgery and ‡Anesthesiology and Reanimation, Osmangazi University, Eskisehir, Turkey
Divisions of †Neurological Surgery and *Neuropathology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
An experimental model is described for producing space-occupying lesions of the spinal cord with kaolin, whose effect on cellular proliferation is well known. Fifteen Sprague-Dawley rats were used in this study. In 10 rats, 0.1 ml (200 mg/ml) kaolin was injected into the posterior subarachnoid space of the thoracic spinal cord at any level between T6 and T10 over 20 seconds. Five control rats were injected with saline in a similar manner. All animals were sacrificed 4 to 5 weeks after the injections, and their spinal cords were harvested for analysis by light microscope. Space-occupying lesions, which appeared to be granulomas, were observed in the posterior spinal cord in five kaolin-injected rats. No gross abnormalities were apparent in the control rats. If a natural space-occupying lesion associated with cellular proliferation in the spinal cord is needed, kaolin-induced granulomas may represent a realistic and reliable experimental model.
Key Words: experimental model, granuloma, kaolin, spinal cord
Kaolin, a ceramic fiber that contains mostly hydroxylated aluminum silicate [Al(4)Si(4)O(10) (OH)(8)], is used as an additive compound in the ceramic industry.8 This refractory clay, whose toxic effect is well known, has been introduced into a variety of tissues to produce inflammation, cellular toxicity, and space-occupying lesions, especially granulomas.12,14,15
In neurological research, kaolin has been used to induce cerebral granulomas, which act as an epileptic foci. In the spinal canal, adhesions produced by kaolin lead to syringomyelia; when instilled in the cisterna magna, it causes hydrocephalus.2-4,7,11,17 Kaolin, however, has not been used in experimental studies to produce intramedullary space-occupying mass lesions.
 Initially, we proposed to produce spinal syrinx cavities by injecting kaolin into the subarachnoid space. Then incidental intramedullary granulomas that might be suitable for spinal cord research were discovered in some rats. Producing mass lesions for spinal cord research must be simple and mimic natural processes if the results are to be generalizable. Some models of extra- and intramedullary compression of the spinal cord have been far from natural nor have they involved cellular space-occupying lesions.1,5 The present kaolin model, however, is a realistic, reliable, and easy method for producing space-occupying lesions involving the spinal cord.
Initially, we proposed to produce spinal syrinx cavities by injecting kaolin into the subarachnoid space. Then incidental intramedullary granulomas that might be suitable for spinal cord research were discovered in some rats. Producing mass lesions for spinal cord research must be simple and mimic natural processes if the results are to be generalizable. Some models of extra- and intramedullary compression of the spinal cord have been far from natural nor have they involved cellular space-occupying lesions.1,5 The present kaolin model, however, is a realistic, reliable, and easy method for producing space-occupying lesions involving the spinal cord.
Material and Methods
After the experimental protocol was approved by and received support from TICAM (Medical and Surgical Research Center of Osmangazi University), 15 Sprague-Dawley rats (300-350 gm) were anesthetized intramuscularly with a xylazine hydrochloride (10 mg/kg) and ketamine (60 mg/kg) mixture. A single- or double-level laminectomy was performed between T6 and T10 under the operating microscope. A special frame designed by TICAM technical service, mounted with a 29-gauge needle and a 10 µl Hamilton syringe, was brought into the operating field. In 10 rats the needle was inserted into the subarachnoid space and 0.1 ml kaolin (200 mg/ml) was injected over 20 seconds. Five control rats had 0.1 ml normal saline injected into the subarachnoid space in the same fashion.
All animals were sacrificed 4 to 5 weeks after injection by transcranial perfusion of 2.5% glutaraldehyde and 2% paraformaldehyde in 0.2 mol/L phosphate buffer (pH 7.4), and the spinal cords from T6 to T10 were quickly removed. Light microscopy specimens were fixed in 2.5% glutaraldehyde, dehydrated in ethanol, and embedded in paraffin. Histological analysis was performed on sections stained with hematoxylin and eosin.
A radiographic fluorescent assay (WRF-8680 ± 266; ARL, Swiss) of the chemical composition of the kaolin (Table 1) showed that approximately 88% (of a total 91.15%) of the compounds in the kaolin were aluminum and silicate.
Results
After the injection, two rats in the kaolin-injected group exhibited weakness of the lower extremities that recovered within 24 to 48 hours. After 48 hours and until they were sacrificed, no animal exhibited a neurological abnormality associated with running, standing, jumping, or responding to painful stimuli. No rat in the control group exhibited a gross abnormality of their spinal cord. Space-occupying lesions were observed in the posterior spinal cord adjacent to the injected area in five rats who had kaolin injected into the posterior subarachnoid space. These gross intraparenchymal lesions were especially close to the injected subarachnoid region.
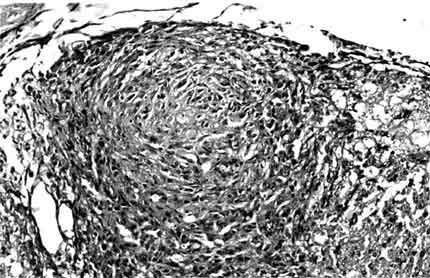
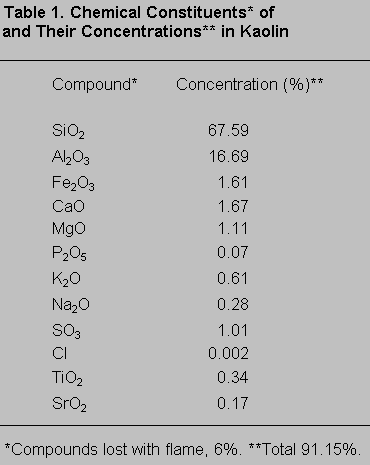
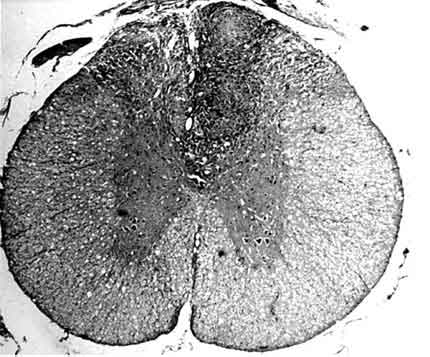
In the posterior portion of the spinal cord, kaolin crystals were traced intraparenchymally. In all rats in the kaolin group, the subarachnoid space was completely obstructed and arachnoidal adhesions were present. Well-formed granulomas were present in the posterior column of five spinal cords (Fig. 1). One of these specimens also had a similar granuloma in the subarachnoid space. The granulomas were primarily composed of spindle-shaped or epithelioid histiocytes and some lymphocytes. Multinucleate histiocytes were not present (Fig. 2). Crystalline foreign material was dispersed throughout the granulomas (Fig. 3). Rather than being infiltrative, the space-occupying lesions were well-demarcated and expansive.
Discussion
Various experimental models 1,6,9,10 for spinal cord compression have been described: inserting implantable artificial masses, suspending tumor cells, and advancing screw or balloon catheters into the spinal canal. Inserting tumor cells into the spinal canal leads to aggressive cellular proliferation that devastates the spinal cord and may kill the animal. Advancing a screw or a balloon catheter into the spinal canal is an unnatural process that fails to mimic a space-occupying lesion of the spinal cord. These methods are extremely different from those associated with space-occupying lesions that occur naturally.
Kaolin has long been introduced into the spinal canal and spinal cord. Although it is known to produce granulomatous inflammation in the spinal canal, its use to produce space-occupying lesions in the spinal cord has not been reported.3,4,11,13 Romero et al.13 have reported that granulomatous leptomeningomyelitis was accompanied by an intense glial reaction after kaolin was injected subdurally in cats. They evaluated the light and electron microscopic aspects of meningeal granulomatous and spinal cord glial reactions but found no evidence of macroscopic spinal cord granulomas. In our study, introducing kaolin into the subarachnoid space led to the formation of intramedullary granulomas adjacent to the injected area in five rats. In earlier experimental models, the doses of the kaolin introduced into the spinal canal were lower than those used in our rat model.3,4,11 The granulomas might therefore have developed because high doses of kaolin (0.1 ml, 200 mg/ml), which are excessive for the small cross-sectional area of a rat’s spinal canal, were used. Higher concentrations of kaolin would likely produce granulomas even more reliably. The kaolin introduced into the subarachnoid space was also traced spontaneously into medullary parenchyma, which then caused cellular proliferation. Mononuclear cellular proliferation in response to intramedullary kaolin produced discrete granulomas in the posterior column. The space-occupying lesions were expansive, compressing rather than infiltrating the surrounding spinal cord parenchyma.
Although the number of rats used in this study was small, the results represent the first indication that spinal cord granulomas that might serve as an acceptable model for a naturally occurring medullary mass can be produced experimentally in an animal. Kaolin could be introduced into various subarachnoid regions of the spinal canal to produce different neurological deficits. With granulomas at various locations in the spinal cord, motor or sensory evoked potentials could be measured to reflect function. In our kaolin-injected group, the abilities to stand and jump were unimpaired on the day of sacrifice. Evaluating the sensory status of rats by clinical observation is fairly difficult. The somatosensory evoked potentials of animals with posterior spinal cord granulomas, however, could be measured as an indication of their somatosensory function.
The spinal cord mass lesions might have formed due to the effect of the various substances in the kaolin, which was primarily composed of aluminum and silicate. Other than the aluminum and silicate, only two other compounds in the kaolin could induce cellular proliferation and inflammation: the titanium dioxide (TiO2) and iron particles.15,16 The concentration of these compounds with kaolin, however, was very low for inducing cellular proliferation and inflammation. Still, these particles might be involved in the formation of the granulomas.
The present kaolin-induced rat model is simple, inexpensive, and easily reproducible with readily available materials. Intramedullary granulomas were produced adjacent to kaolin-injected regions of the subarachnoid space. Hence, such granulomas could likely be produced in any desired region of the spinal cord. The model offers researchers the potential to evaluate any region of the spinal cord and closely mimics the natural formation of intramedullary tumor lesions. It may be useful for further studies of the pathogenesis of space-occupying lesions of the spinal cord. If a natural space-occupying lesion is desired, spinal cord granulomas can be produced by injecting high doses of kaolin.
References
- Arbit E, Galicich W, Galicich JH, et al: An animal model of epidural compression of the spinal cord. Neurosurgery 24:860-863, 1989
- Calvillo M, Paz C, Sotelo J: Changes in the threshold of pentylenetetrazol-induced seizures in cats with a chronic granuloma in brain amygdala. Epilepsy Res 16:45-49, 1993
- Cho KI, Iwasaki Y, Imamura H, et al: Experimental model of posttraumatic syringomyelia: The role of adhesive arachnoiditis in syrinx formation. J Neurosurg 80:133-139, 1994
- De Estable-Puig RF, Estable-Puig JF, Romero C, et al: Pathological study of the cat spinal cord after chronic local implantation of aluminum hydroxide. II. Electron microscopic study of the meningeal granuloma and spinal meningeal borderline. Exp Pathol (Jena) 9:288-301, 1974
- Doppman JL, Ramsey R, Thies RJ, II: A percutaneous technique for producing intraspinal mass lesions in experimental animals. J Neurosurg 38:438-447, 1973
- Griffiths IR: Vasogenic edema following acute and chronic spinal cord compression in the dog. J Neurosurg 42:155-165, 1975
- Hatton JD, Ellisman MH: Orthogonal arrays are redistributed in the membranes of astroglia from alumina-induced epileptic foci. Eplepsia 25:145-151, 1984
- Herman SJ, Olscamp GC, Weisbrod GL: Pulmonary kaolin granulomas. J Can Assoc Radiol 33:279-280, 1982
- Hukuda S, Wilson CB: Experimental cervical myelopathy: Effects of compression and ischemia on the canine cervical cord. J Neurosurg 37:631-652, 1972
- Ikeda H, Ushio Y, Hayakawa T, et al: Edema and circulatory disturbance in the spinal cord compressed by epidural neoplasms in rabbits. J Neurosurg 52:203-209, 1980
- Milhorat TH, Nobandegani F, Miller JI, et al: Noncommunicating syringomyelia following occlusion of central canal in rats. J Neurosurg 78:274-279, 1993
- Murphy EJ, Roberts E, Horrocks LA: Aluminum silicate toxicity in cell cultures. Neuroscience 55:597-605, 1993
- Romero C, Estable-Puig JF, De Estable-Puig RF, et al: Pathological study of the cat spinal cord after chronic local implantation of aluminum hydroxide. Exp Pathol (Jena) 6:333-342, 1972
- Sluka KA, Rees H, Westlund KN, et al: Fiber types contributing to dorsal root reflexes induced by joint inflammation in cats and monkeys. J Neurophysiol 74:981-989, 1995
- Stenback F, Rowland J, Sellakumar A: Carcinogenicity of benzo(a)pyrene and dusts in the hamster lung (instilled intratracheally with titanium oxide, aluminum oxide, carbon and ferric oxide). Oncology 33:29-34, 1976
- Warheit DB, Hansen JF, Yuen IS, et al: Inhalation of high concentrations of low toxicity dusts in rats results in impaired pulmonary clearance and persistent inflammation. Toxicol Appl Pharmacol 145:10-22, 1997
- Yamada H, Yokota A, Haratake J, et al: Morphological study of experimental syringomyelia with kaolin-induced hydrocephalus in a canine model. J Neurosurg 84:999-1005, 1996
