
Pediatric MR Imaging
Authors
Ameet Patel, MD
C. Roger Bird, MD
Erin Prenger, DO
Division of Neuroradiology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
Neuroradiologic imaging has seen marked changes in the past decade. Much of the advancement in imaging has been the development of improved magnetic resonance (MR) imaging hardware and software. This technology has facilitated the development of new pulse sequences that have improved the sensitivity and specificity of imaging. The application of these technologies to pediatric imaging has improved identification and treatment planning. This article reviews some of the advances in MR imaging in the past decade, specifically diffusion imaging, MR spectroscopy, and cine imaging, and describes their application to pediatric patients.
Key Words: cine imaging, diffusion imaging, magnetic resonance spectroscopy, pediatric magnetic resonance imaging
Magnetic resonance (MR) imaging has markedly improved diagnostic accuracy in pediatric patients. In MR imaging, tissue characterization is based on the inherent T1- and T2-weighted relaxation values of tissues. The mainstays of MR imaging, consisting of T1-, intermediate-weighted, and T2-weighted images, provide more conspicuous visualization of tissue characteristics than computed tomography (CT). This statement is especially true for white matter lesions, which previously were not depicted well by CT. In the early days of MR imaging, the most dramatic improvements were in the depiction of the anatomic detail of the brain and its abnormalities.
Recent advances in MR imaging have focused on functional aspects of the brain. In the past decade, many new pulse sequences with which to examine the brain have been developed. Each sequence has strengths and weaknesses that make it uniquely sensitive to certain pathologic states, allowing identification of abnormalities that may be subtle or invisible on routine T1- and T2-weighted MR imaging. Advances in imaging have also improved the depiction of the normal patterns of myelination. Sequences may be combined to tailor an examination to answer specific questions and to help clinicians manage patients. Diffusion imaging, MR spectroscopy, cine imaging, and functional imaging are but a few of the techniques that have gained widespread clinical utility in the past decade.
Diffusion-Weighted Imaging
Stroke may be defined as an acute loss of neurologic function caused by an ischemic injury or hemorrhage. Stroke is less common among children than adults. Hemorrhagic strokes comprise a smaller fraction of strokes in children than in adults. In children, ischemic strokes can be caused by vasculitis, sickle cell anemia, an intracranial infection such as meningitis, hypotension, thromboembolic phenomenon (e.g., cardiac, fibromuscular dysplasia, or dissection), or metabolic abnormalities (homocystinuria). Typically, ischemia in children is acute and seldom chronic.
Intracranial hemorrhage, predominantly germinal matrix hemorrhage in neonates, has been well studied by transcranial ultrasonography. Primarily CT or MR imaging has been used to image ischemic strokes in children. Although CT can define ischemic or infarcted brain, findings can be detected earlier on MR imaging, which is more sensitive and specific than CT. On CT, hypodensity—or the loss of the interface between gray and white matter—is visible once edema develops many hours after the ischemic event. T2-weighted MR imaging can detect cytotoxic edema, which forms earlier. Abnormalities on T2-weighted imaging have been identified on the order of several hours after the ictus. Contrast-enhanced MR imaging can detect strokes before T2-weighted changes develop. Enhancement of arteries, especially major arteries, after contrast administration suggests sluggish blood flow.[17] Normally, there should be no signal (flow-void) within larger arteries and veins related to dephasing of protons from motion in a magnetic field. Later in the course of a stroke, edema, sulcal effacement, and mass effect with a midline shift may become evident.
The abnormally increased intensity of T2-weighted signals in an ischemic or infarcted brain is a nonspecific finding for ischemia. Other causes such as encephalitis, gliosis, or malignancy can also increase the intensity of T2-weighted signals. Furthermore, the normally decreased intensity of T1- and increased intensity of T2-weighted signals in the white matter of infants, reflecting their immature pattern of myelination, makes the detection of a T2-weighted abnormality more difficult than in adults. A new technique, referred to as diffusion-weighted imaging, is a powerful tool for detecting acute ischemic strokes and is more sensitive and specific than T2-weighted MR imaging. Diffusion is also useful for detecting encephalitis and for differentiating arachnoid cysts from epidermoids. In the future, it may also help differentiate dysmyelinating diseases from demyelinating diseases.[6]
Diffusion-weighted imaging detects the translational motion of water molecules in vivo. In MR imaging a 180º refocusing pulse is used to realign the phase of dephased spinning protons (only in-phase protons can generate a measurable signal). In diffusion-weighted imaging, a powerful gradient is applied before the 180º pulse, and a second identical gradient is applied after the 180º pulse. Because of the intervening 180º pulse, the two gradients cancel each other as long as protons do not move.
If the protons move between the first and second gradient application, however, they will not become refocused and will not generate a measurable signal. The dephasing of protons is proportional to the distance traveled by the protons and to the strength of the applied gradient field. Consequently, this motion can be quantitated by measuring the amount of dephasing that protons moving parallel to the applied gradient undergo relative to stationary protons. This method thereby determines which protons are free to move (little or no signal) and which ones are restricted in their motion (increased signal).
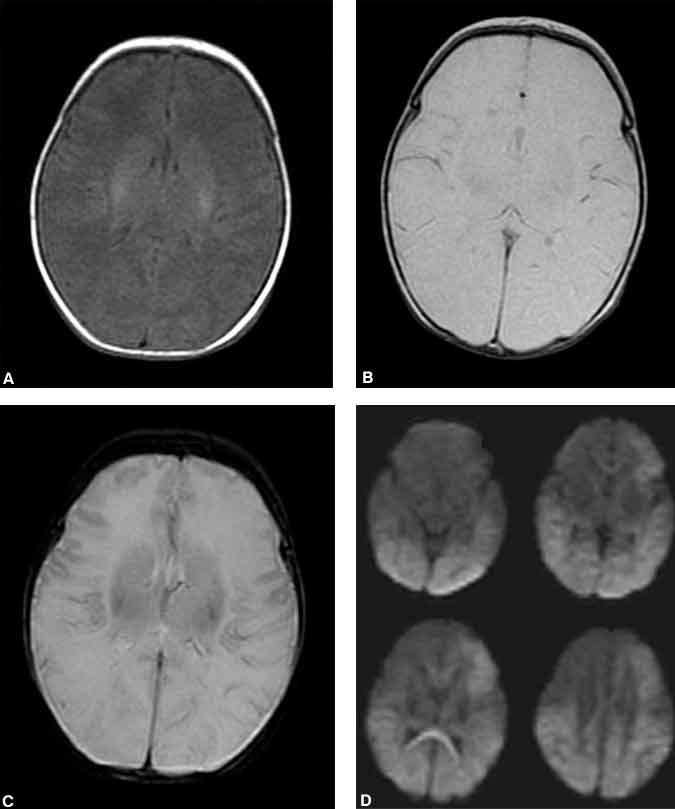
This freedom of motion can be quantitated and represented as an apparent diffusion coefficient map. A more clinically useful image of the brain can be generated by presenting areas of restricted motion as bright pixels and areas of unrestricted motion as dark pixels. This diffusion imaging sequence has only recently become possible with the development of MR image scanners and gradient coils in particular capable of generating gradients powerful enough to be sensitive to the minute and rapid diffusion motion of water molecules.
Diffusion-weighted MR images provide a powerful tool for the detection of acute ischemic changes. Once a volume of brain has reached a threshold of ischemia (decreased oxygen or glucose), the production of high-energy substrates such as adenosine triphosphate decreases below the consumption level for these substrates. The Na+_K+ pump starts to fail, and the neuron is unable to maintain the high electrical potential across its cell membrane necessary for depolarization. Although not dead, the neuron becomes functionally silent.
With further ischemia the neuron becomes incapable of maintaining the integrity of its cell membrane, and it swells from the influx of sodium and water. Although the total volume occupied by ischemic brain may not have changed (a T2-weighted abnormality), the movement of extracellular fluid into the intracellular compartment decreases the overall freedom of movement for water molecules. The mechanism is not completely elucidated, but this restriction of movement is thought to underlie abnormal signals on diffusion-weighted images. Ischemic areas of brain associated with the restricted motion of water molecules appear hyperintense on diffusion-weighted images of the brain (Fig. 1).
Much of the literature on diffusion-weighted imaging in strokes has been based on studies in adults. In adult animal models, abnormal diffusion signals can be identified within minutes of arterial occlusion. In adult humans abnormal diffusion signals have consistently been identified before T2-weighted abnormalities and have been demonstrated as soon as 1.5 hours after symptom onset.
In animals, abnormal diffusion signals have been reversible. Initial experience in adult humans has indicated that diffusion abnormalities evolve into infarctions, but recent examples have shown some reversibility. Our data indicate that diffusion abnormalities normalize 14 days after an ischemic event in almost all adults. T2-weighted abnormalities remain hyperintense indefinitely after an ischemic event. Therefore, diffusion abnormalities can be used to differentiate between infarctions less than 2 weeks old and other causes of T2-weighted abnormalities.
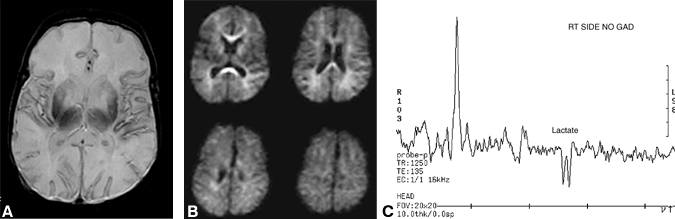
Diffusion-weighted abnormalities in children, particularly in infants, are not as well studied as in adults, but much of the literature supports the notion that diffusion imaging in infants is similar to that in adults. In our experience, diffusion-weighted imaging is not as robust in neonates with a hypoxic-ischemic injury as it is in adults with infarcts. In some of our cases diffusion-weighted images were normal while intermediate- or T2-weighted images showed subtle abnormalities that were later confirmed as areas of infarction (Fig. 2). Abnormalities on diffusion-weighted images of infants with ischemia related to arterial abnormalities, such as embolic or thrombotic occlusion, follow a pattern similar to that of adults. Diffusion studies from infants with hypoxic-ischemic injury caused by more global hypoperfusion initially may appear normal.
Studies of the immature brain during ischemia performed in a rat model have shown increases in the intensity of both diffusion-weighted and T2-weighted signals simultaneously.11 This abnormality improves soon after the ischemic event, and a secondary, delayed increase in the intensity of both the diffusion-weighted and T2-weighted signals follows. During this evolution, there may be an interval in which the T2-weighted signal abnormality exceeds the diffusion-weighted abnormality. This pattern of signal abnormality has not been demonstrated in adults.
Although diffusion-weighted imaging has found most widespread clinical utility in the imaging of acute stroke, it has also been useful in investigating other abnormalities such as differentiating arachnoid cysts from epidermoids. Both entities are extra-axial lesions found in children and adults. Both appear hypointense on T1-weighted and hyperintense on T2-weighted images and do not enhance. On all pulse sequences, the signal from arachnoid cysts follows that from cerebrospinal fluid (CSF). Epidermoid tumors tend to be slightly hyperintense compared to CSF on T1-weighted and proton-density images. However, they may appear isointense on all pulse sequences, making them difficult to differentiate from arachnoid cysts. On diffusion-weighted images arachnoid cysts are, like CSF, dark. In contrast, epidermoids are markedly hyperintense.10 We have found no exceptions to these characteristics. Therefore, diffusion-weighted imaging has provided a level of tissue characterization not previously possible with routine T1- and T2-weighted imaging.
Diffusion-weighted imaging has also been useful in the detection of viral infections of the brain. Although encephalitis can have a variety of causes, it is typically caused by a viral infection, most commonly from the Herpes simplex virus. Early detection and treatment are essential to achieve good neurologic outcomes. Diffusion-weighted imaging is more sensitive for the detection of early Herpes encephalitis than conventional T1- or T2-weighted imaging, or even fluid-attenuated inversion recovery imaging. Hyperintensity on diffusion-weighted imaging is thought to represent cytotoxic edema and can be detected before a T2-weighted abnormality becomes apparent. The imaging abnormalities resolve with successful treatment.[9]
Spectroscopy
Scientists use MR spectroscopy to elucidate the structure of molecules. The varying chemical environment of each nucleus in a complex organic molecule results in minute variations in the local magnetic field experienced by each nucleus. This inhomogeneity in the magnetic field causes slight differences in the Larmor frequency of the nucleus, which can be measured and displayed as a graph or “spectra.” Variation in the Larmor frequency is referred to as chemical shift.
The chemical structure of a compound and hence its identity can be deduced from graphs of these chemical shifts, allowing greater tissue characterization than is possible with routine MR imaging alone. Several magnetic nuclei such as phosphorus, sodium, carbon, fluorine, lithium, and hydrogen can be imaged by MR spectroscopy. Protons are the most widely imaged particle because of their great abundance in the brain and their great nuclear magnetic sensitivity. Proton spectroscopy can also be performed with conventional head coils.
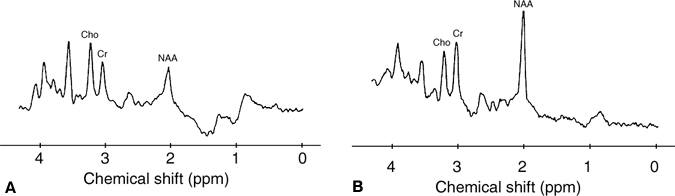
Until recently, MR spectroscopy has rarely been applied clinically. The availability of commercial software spectroscopy packages, however, has increased its usage, and ongoing studies are further defining its clinical utility. In pediatric patients, MR spectroscopy has been used to evaluate neurodegenerative diseases with variable success (Fig. 3). Degenerative brain disorders such as Leigh’s, Pelizaeus-Merzbacher, Alexander’s, Cockayne’s, and Canavan’s diseases are often associated with nonspecific clinical and imaging findings. An abnormal T2-weighted signal in the context of these diseases is a nonspecific finding whose detection is complicated by the normally “wet” brain of infants.
MR spectroscopy has found elevated peaks for n-acetyl aspartate (NAA) and myoinositol in children with Canavan’s disease. NAA is not known to be elevated in any other disease and so provides a relatively specific finding for Canavan’s disease.[2,14] This elevation is caused by a deficiency of aspartoacylase, which causes NAA to accumulate.
Although MR spectroscopy has been more disappointing in the differentiation and classification of neurodegenerative diseases, demonstrating a nonspecific decreased peak for NAA and an elevated peak for lactate, it has shown promise for the early detection of disease activity and possibly for monitoring the course of a disease as well.[3,12,13] Demyelinating processes are associated with a decrease in the peak of NAA. In children with adrenoleukodystrophy, the NAA/ creatine ratio is decreased and the choline/creatine ratio is elevated. Elevated choline levels may be a marker for active demyelination. Lactate, glutamate, glutamine, and inositol may also be elevated in these patients.[7,13]
A rare disorder involving mitochondrial myopathy, encephalopathy, lactic acidosis, and strokes is known as MELAS. In these patients, the lactate peak (identified by the characteristic inverted doublet when an echo delay TE of 135 ms is used) is elevated. Cerebral infarctions are also associated with elevated lactate peaks (Fig. 2), but the levels normalize over time unlike in MELAS.
Despite its lack of diagnostic specificity, MR spectroscopy may be useful for monitoring disease activity. Maple syrup urine disease causes branched-chain amino acids to accumulate due to a defect in oxidative decarboxylation of branched-chain ketoacids. These children have sporadic decompensation from cerebral edema. MR spectroscopy has demonstrated a reversible decrease in the NAA/creatine ratio and an elevated lactate peak during the decompensation. The lactate peak correlates with cerebral edema, which correlates with the patient’s condition. A new peak, identified at approximately 1 ppm, is presumed to represent the branched-chain amino acids.[3,14]
The clinical application of MR spectroscopy is still in an early stage of development, but its approval for clinical use by the Food and Drug Administration should encourage progress. As experience with the spectra of various disease states increases and technology advances, MR spectroscopy will likely become a powerful tool enabling specific tissue characterization and helping clinicians to diagnose diseases and to monitor treatment efficacy.
Seizures are episodes of abnormal cerebral activity caused by the excessive discharge of cortical neurons. There are approximately 100 cases of epilepsy per 100,000 individuals in the first year of life. Thereafter, the incidence markedly decreases until age 50 at which time the incidence starts to increase until it reaches 180 cases per 100,000 individuals by the age of 85 years.
Epilepsy is caused by a wide variety of lesions such as prior trauma or hemorrhage, cortical dysplasias, and benign or malignant brain tumors. Often, the seizure focus is identified while a cause is not. Partial complex seizures often arise from the temporal lobes. In such patients, resection of the anterior temporal lobe may markedly improve their condition with minor risk.
The accurate identification and lateralization of the epileptogenic focus are essential to select patients most likely to benefit from surgery. The utility of MR imaging, scalp and depth electrode mapping, positron emission tomography, and single photon emission computed tomography varies for lateralizing a seizure focus. High-resolution imaging of the temporal lobes may demonstrate an abnormal T2-weighted signal and decreased volume in the hippocampi, suggesting the presence of mesial temporal sclerosis and thus lateralizing the epileptogenic focus. MR imaging, however, reveals no abnormality in many patients with temporal lobe epilepsy. A more sensitive and reliable method of identifying the side of the abnormality would improve the management of some of these patients.
MR spectroscopy has identified biochemical abnormalities in the brains of patients with epilepsy. Proton spectroscopy in patients with temporal lobe epilepsy is in an early stage of development, but preliminary results are promising. Most studies evaluate the concentrations of NAA, choline, creatine, and lactate. Other organic compounds such as glutamine, glutamate, and inositol may also be measured. All studies thus far have described a decrease in the peak of NAA in the temporal lobe of affected patients compared to normal controls.
Most patients also demonstrate a decrease in the peak of NAA compared to the contralateral temporal lobe. Some patients (approximately 20%), however, have a bilateral abnormality. In fact, more patients may have a bilateral abnormality than previously suspected. In affected temporal lobes, pathologic studies have demonstrated neuronal loss and gliosis, which the decreased NAA peak is thought to reflect. Several investigators have also found a decrease in the NAA/creatine or NAA/(creatine + choline) ratio, but not all investigators have found changes in the creatine or choline peaks.[8]
Phosphorus MR spectroscopy can be used to study energy levels in the brain and has been used to study patients with epilepsy. Elevated pH levels have consistently been identified in epileptogenic foci in patients with temporal lobe and frontal lobe seizures. Elevated levels of inorganic phophate and decreased levels of phosphomonoesters have been identified not only in the epileptogenic focus in the temporal lobe but also in the adjacent temporal lobe. In contrast, frontal lobe epileptogenic foci do not demonstrate abnormal levels of inorganic phosphate.
Phosphorus MR spectroscopy has not found widespread clinical use. Technically, it is difficult. It also requires a dedicated head coil tuned to the precession frequency of phosphorus. Additionally, images are signal starved because of the small concentrations of phosphorus and because the nuclear magnetic sensitivity of phosphorus is less than that of protons.
Functional MR imaging is another emerging tool applied to patients with epilepsy. Diffusion-weighted imaging, a subcategory of functional imaging, has already been discussed. Perfusion imaging and blood oxygen level dependent (BOLD) imaging are two other examples of functional imaging. Perfusion-weighted imaging focuses on blood flow at the capillary level and involves either exogenous contrast administration or endogenous labeling techniques. BOLD imaging techniques have been applied more frequently in patients with epilepsy than perfusion techniques. BOLD imaging paradigms are varied but typically require patients to perform a task repeatedly, whether mental or physical, while the brain is scanned for changes in perfusion. In this manner the location of the activity in the brain, reflecting the local increase in perfusion associated with cortical activation, can be mapped.
Functional MR imaging has not been particularly useful in identifying epileptogenic foci. However, it has shown some promise in lateralizing speech and memory and in the presurgical mapping of eloquent brain. Currently, a Wada test is used preoperatively to identify patients with epilepsy who are at high risk for developing postoperative memory deficits and to lateralize speech function.[15] The Wada test is invasive and entails the selective injection of amybarbital into the internal carotid arteries. The technique exposes patients to the inherent risks of angiography and is contraindicated in those with persistent fetal circulation because of the risk of anesthetizing the brain stem.
Binder et al.[1] found a strong correlation between BOLD functional imaging and Wada testing for the hemispheric lateralization of speech function in 22 patients with epilepsy. Preoperative memory testing in patients with mesial temporal sclerosis is important to avoid postoperative anterograde amnesia. Mapping memory with functional MR is more difficult than language lateralization. Consequently, Wada testing is still used to evaluate patients with complex partial seizures localized to the temporal lobes. Further development of functional imaging may preclude the need to expose patients to the risks of a Wada test.
Patients who are to undergo cortical resection to remove an epileptogenic focus or tumor may also benefit from functional MR mapping of the adjacent eloquent brain. Preoperative mapping permits a more informed assessment of the surgical risks to patients and more accurate surgical planning.[15]
Cortical dysplasias, either heterotopias or incomplete lissencephalies, are another cause of seizures. MR imaging has improved the detection of these congenital abnormalities immensely. Nevertheless, subtle abnormalities still escape detection by conventional MR imaging. The application of ultrahigh-resolution MR imaging using three-dimensional (3D) acquisitions can help identify such abnormalities. The entire brain, however, cannot yet be imaged by these time-consuming techniques. These techniques are most useful when directed to a small region of the brain by other tests such as electroencephalography.
Cine Imaging
Hydrocephalus is the dilatation of the ventricular system related to discrepancies between CSF production and resorption. In infants with hydrocephalus, the sutures often separate or head circumference enlarges. Clinically, infants may present with lethargy, headache, and vomiting. Hydrocephalus can lead to developmental delays and mental retardation.
Imaging studies cannot always reliably differentiate between hydrocephalus and other causes of ventricular dilatation such as loss of neuronal volume. The relative dilatation of the temporal horns and of the anterior and posterior recesses of the third ventricle is considered a relatively reliable indicator of hydrocephalus.
The etiology of hydrocephalus includes aqueductal stenosis (congenital and postinfectious), tectal gliomas, meningitis, and subarachnoid hemorrhage. Regardless of etiology, an alternate pathway of CSF drainage must be provided. Treatment options include traditional shunting procedures from the ventricular system to the peritoneum or a third ventriculostomy in selected patients. The third ventriculostomy is most useful when the obstruction occurs at the aqueduct of Sylvius. It involves the formation of a communication between the floor of the third ventricle and the suprasellar cistern, thereby bypassing the obstruction.
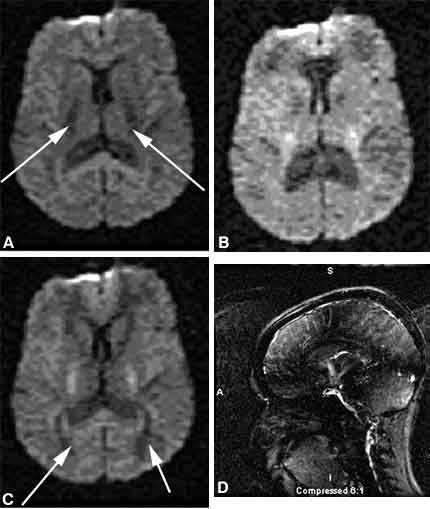
Previously, patency or obstruction of the shunt had to be inferred from the stability or dilatation of the ventricles over time. In some patients the clinical symptoms may change without detectable changes in ventricular size. The patency of the shunt can be assessed using a new MR imaging technique called cine imaging. When a shunt is patent, midline sagittal cine images demonstrate to-and-fro flow, displayed as a jet of black or white in the suprasellar cistern (Fig. 4), compared to the relatively homogenous gray of the brain. Fukuhara et al.4 have demonstrated good correlation between the lack of visualization or visualization of subtle CSF flow at the third ventriculostomy site and obstruction or near obstruction of the third ventriculostomy. The visualization of good CSF flow correlated well with patency of the third ventriculostomy as proven by reexploration.[4]
Hydrocephalus in children with an Arnold-Chiari malformation can also be studied using cine techniques. Hydrocephalus accompanies the Arnold-Chiari malformation in 20 to 25% of Chiari I patients. In the past decade cine imaging has advanced our understanding of the pathophysiologic mechanisms underlying the Chiari I malformation. Conflicting reports describe abnormalities of CSF flow and pulsation at the foramen magnum in patients with a Chiari I malformation. Most studies, however, demonstrate abnormal motion of the cerebellar tonsils and upper spinal cord in these patients. Some postoperative studies have also documented improved CSF flow after decompression. Incomplete knowledge of the pathophysiology, however, precludes using these flow data for therapeutic decisions. We have used cine imaging to evaluate CSF flow around the brainstem and cerebellar tonsils.[5,16]
References
- Binder JR, Swanson SJ, Hammeke TA, et al: Determination of language dominance using functional MRI: A comparison with the Wada test. Neurology 46:978-984, 1996
- Castillo M, Kwock L, Mukherji SK: Clinical applications of proton MR spectroscopy. AJNR Am J Neuroradiol 17:1-15, 1996
- Felber SR, Sperl W, Chemelli A, et al: Maple syrup urine disease: Metabolic decompensation monitored by proton magnetic resonance imaging and spectroscopy. Ann Neurol 33:396-401, 1993
- Fukuhara T, Vorster SJ, Ruggeri P, et al: Third ventriculostomy patency: Comparison of findings at cine phase-contrast MR imaging and at direct exploration. AJNR 20: 1560-1566, 1999
- Hofmann E, Warmuth-Metz M, Bendszus M, et al: Phase-contrast MR imaging of the cervical CSF and spinal cord: Volumetric motion analysis in patients with Chiari I malformation. AJNR 21: 151-158, 2000
- Ono J, Harada K, Mano T, et al: Differentiation of dys- and demyelination using diffusional anisotropy. Pediatr Neurol 16:63-66, 1997
- Rajanayagam V, Grad J, Krivit W, et al: Proton MR spectroscopy of childhood adrenoleukodystrophy. AJNR Am J Neuroradiol 17:1013-1024, 1996
- Thompson JE, Castillo M, Kwock L: MR spectroscopy in the evaluation of epilepsy. Magn Reson Imaging Clin N Am 6:21-9, 1998
- Tsuchiya K, Katase S, Yoshino A, et al: Diffusion-weighted MR imaging of encephalitis. AJNR Am J Neuroradiol 173:1097-1099, 1999
- Tsuruda JS, Chew WM, Moseley ME, et al: Diffusion-weighted MR imaging of the brain: Value of differentiating between extraaxial cysts and epidermoid tumors. AJNR Am J Neuroradiol 11:925-934, 1990
- Tuor UI, Kozlowski P, Del Bigio MR, et al: Diffusion- and T2-weighted increases in magnetic resonance images of immature brain during hypoxia-ischemia: Transient reversal posthypoxia. Exp Neurol 150:321-328, 1998
- Tzika AA, Ball WS, Jr., Vigneron DB, et al: Clinical proton MR spectroscopy of neurodegenerative disease in childhood. AJNR Am J Neuroradiol 14:1267-1284, 1993
- Tzika AA, Ball WS, Jr., Vigneron DB, et al: Childhood adrenoleukodystrophy: Assessment with proton MR spectroscopy. Radiology 189:467-480, 1993
- Want ZJ, Zimmerman RA: Proton MR spectroscopy of pediatric brain metabolic disorders. Neuroimaging Clin N Am 8:781-807, 1998
- Weiss KL, Figueroa RE, Allison J: Functional MR imaging in patients with epilepsy. Magn Reson Imaging Clin N Am 6:95-112, 1998
- Wolpert S, Bhadelia R, Bogdan A, et al: Chiari I malformations: Assessment with phase-contrast velocity MR. AJNR 15: 1299-1308, 1994
- Yuh WT, Crain MR, Loes DJ, et al: MR imaging of cerebral ischemia: Findings in the first 24 hours. AJNR Am J Neuroradiol 12:621-629, 1991
