Perception of Facial Affect After Acute Brain Injury
Lanie Y. Zigler, PhD
George P. Prigatano, PhD
Division of Neurology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
As part of the BNI Screen for Higher Cerebral Functions (BNIS), 100 acutely impaired brain dysfunctional patients were asked to identify verbally three facial affects (happy, angry, fear-surprise). Compared to 3% of 100 normal controls, 40% of the brain dysfunctional patients demonstrated errors in their perception of facial affect (c2=40.5, df=1, p<.001). Errors made by the brain dysfunctional patients tended to be negative or pejorative (i.e., facial affects seen as unwanted or disgusted; 38%) or reflected confusion or uncertainty (33%). Assessing the qualitative errors that brain dysfunctional patients make in the perception of facial affect may provide information concerning their phenomenological state. This information may have diagnostic and rehabilitative utility.
Key Words : affect, brain injury, perception
The perception of facial affect (or emotion) is vital for survival [7] and can be disturbed by various brain disorders.[10] Seldom, however, is this ability assessed by existing neuropsychological tests or examined for its relationship to rehabilitation outcomes. Recently, Prigatano and Wong[21] noted that recovery of emotional functions were as strongly related to the achievement of inpatient rehabilitation goals as cognitive recovery. Given this finding and other observations,[15] we evaluated whether acutely brain dysfunctional patients experience difficulty in perceiving basic affects correctly (happy, angry, fearful) and whether lesion location (as documented by early computed tomography [CT] and magnetic resonance [MR] imaging) related to errors in perception. The study also explored the qualitative errors that brain dysfunctional patients make when asked to perceive facial affect. Such errors may reflect the patients’ phenomenological state[21,24] and provide useful information for rehabilitation planning. No hypothesis was made about the nature of the qualitative errors, but the errors were classified as they naturally occurred. It has been our clinical impression that many severely impaired patients have difficulty with the perception of facial affect, irrespective of the location of their lesion.
Methods
Instrument
The BNI Screen for Higher Cerebral Functions (BNIS) is a brief neuropsychological screening test with demonstrated reliability and validity for identifying brain dysfunctional individuals.19 It was developed to assess patients at various stages after brain injury and is administered to patients at our institution soon after onset of a brain lesion, and then several weeks and months after injury.

The test requires 15 to 30 minutes to administer, depending on the patient’s level of impairment. Scoring is based on the maximum raw score of 50 points, which is converted into a T-score based on age norms.* The BNIS assesses seven neuropsychological constructs: speech and language, orientation, attention/concentration, visuospatial, memory, affect, and awareness. The BNIS measures the construct of affect on four dimensions: affect expression, affect perception, affect control, and spontaneous affect. This study focused on the perception of facial affect only. To assess the perception of facial affect on the BNIS, subjects are asked to identify verbally the feeling expressed on each of three faces (angry, happy, and fear/surprise; Fig. 1). Subjects received a score of either 0 (at least 1 incorrect) or 1 (all correct). Total BNIS scores, calculated as an age-corrected T-score, were also calculated for each patient.
Subjects
Experimental subjects consisted of 100 patients (age range, 18 to 84 years) consecutively admitted to the Barrow Neurological Institute after acute brain injury who were assessed using the BNIS. Patients were either on acute neurology units of the Barrow Neurological Institute or on the Inpatient Neurorehabilitation Unit at St. Joseph’s Hospital and Medical Center. All patients underwent either CT, MR imaging, or both. Patients were evaluated a mean of 17.6 days after lesion onset (standard deviation [SD]=11.48). Their injuries reflected many etiologies: cerebrovascular accidents (48%), traumatic brain injuries (32%), tumors (5%), aneurysm clipping (4%), hydrocephalus (3%), encephalitis (3%), meningitis (2%), transient ischemic attacks (1%), seizures (1%), and gunshot wounds to the head (1%). One hundred age-matched controls who were studied as a part of the standardization of the BNIS served as control subjects.[19] The control subjects were selected to match the brain dysfunctional patients for age, education, handedness, and gender.
Statistical Analysis
All statistical analyses were performed with the Statistical Package for the Social Sciences (version 8.0). Descriptive statistics were obtained for both experimental and control subjects. Affect perception by control and experimental subjects and the effect of injury location (experimental subjects only) on affect perception were analyzed by Chi square tests. Age, gender, education, and BNI total T-score conversions between experimental subjects and controls and between subjects scoring either “0” (a failing score) or “1” (a passing score) on affect perception were analyzed by t tests. A frequency analysis of qualitative error types was also calculated.
Results
 Control Versus Experimental Subjects
Control Versus Experimental Subjects
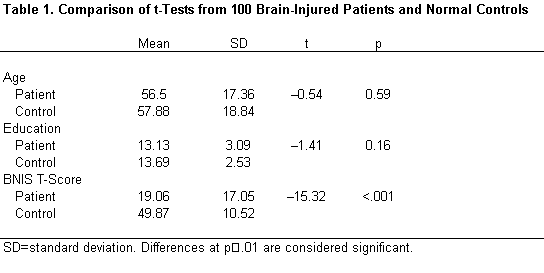
Matching precluded significant differences between controls and experimental subjects on the variables of age, education, or gender (Table 1). As expected, experimental subjects had significantly lower total BNIS T-scores than control subjects (t=–15.32, p<.001; Table 1). The scores of brain dysfunctional patients were three SDs below the mean, indicating severe impairments as a group.
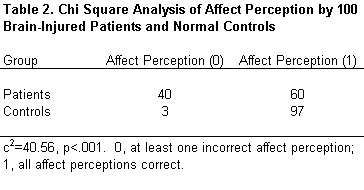
The accuracy of perception of facial affect was substantially worse in patients than in controls (c2=40.56, p <.001). Forty percent of patients with an acute brain disorder failed this task compared to only 3% of the normal controls (Table 2).
 Analysis Within Experimental Subjects
Analysis Within Experimental Subjects
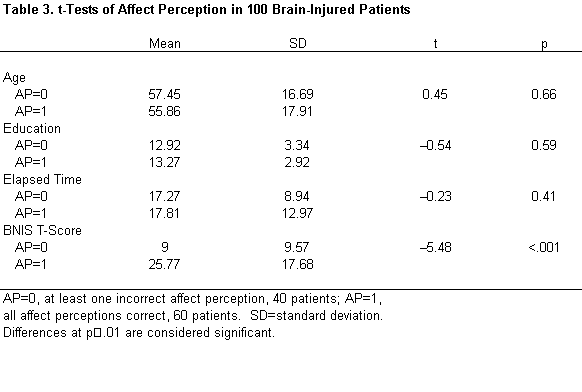
Brain dysfunctional patients scoring “0” were compared to those scoring “1” on the measure of affect perception. There were no significant differences between these groups in terms of age, education, time since injury, or gender, but their BNIS T-scores did differ significantly ( t =–5.48, p <.001; Table 3). The more impaired patients made more errors in the perception of facial affect. Of the patients who had a low BNIS score (T<35), half failed to perceive facial affect accurately. In contrast, no patient with a BNIS T score higher than 35 failed to perceive facial affect accurately.
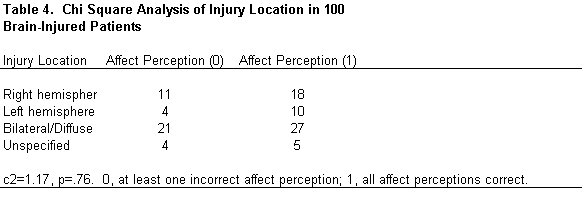
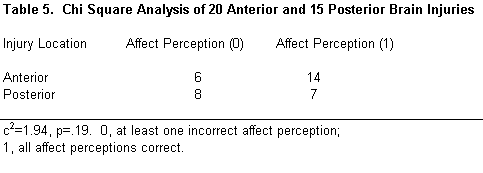
Lesion location was grouped into four categories: right, left, bilateral/diffuse, and unspecified. Lesion location did not differ significantly among subjects (c 2 =1.17, p =.76; Table 4). Nor did an anterior or posterior lesion location significantly affect performance on the perception of affect (c 2 =1.94, p =.19). Only patients whose lesions were clearly anterior or posterior were included in this analysis (Table 5).
Qualitative Analysis of Perception of Facial Affect Errors
Most brain dysfunctional patients could perceive happy (94%) and angry faces (96%) accurately. Only 64% of the brain dysfunctional patients, however, accurately perceived the more ambiguous fearful/surprised face. Of the qualitative errors made, 38% were clearly negative or pejorative (i.e., the face was described as unwanted or as showing disgust). In 33% of the errors, the face was described as being confused or as showing uncertainty. In 13% of the errors, the face was described with neutral terminology (so-so or nonchalant). In 11% of the errors, the face was actually perceived as positive (as happy or pleasant). In 5% of the cases, patients stated that they “didn’t know” what facial affect was characterized by the fearful or surprised face.
Discussion
Cross-cultural studies have demonstrated the universality of at least six facial expressions or emotions (i.e., happiness, sadness, surprise, fear, anger, and disgust). Noninjured and brain-injured individuals, however, do not perceive all emotions the same. The work of Ekman and Friesen[8] suggested that happy, angry, and fearful (or surprised) are among the basic facial emotions that can easily be perceived across cultures. Statistically, normal individuals have little difficulty perceiving happy or angry faces correctly. Fearful faces are often perceived accurately but are more difficult to identify correctly. A fearful face is often perceived as surprised. For example, people from Papau, New Guinea could perceive happy faces with an accuracy of 95 to 100% and angry faces with an accuracy of 67 to 96%.[8] In contrast, a fearful face was perceived correctly only 54 to 85% of the time and was often misjudged as reflecting surprise.
Brain dysfunctional patients may show a similar pattern. They are able to distinguish happy from angry faces but have more difficulty perceiving fearful faces correctly.[12] After encephalitis, individuals exhibit more difficulty perceiving fearful faces correctly than other facial affects.[5]
Our patients’ overall level of neuropsychological functioning, as measured by the total BNIS score, was related to errors in the perception of facial affect. Half of the patients with a BNIS total score less than 35 failed to perceive facial affect accurately. In contrast, no patient with a BNIS T-score above 35 failed to perceive facial affect accurately. This finding suggests that level of cognitive impairment contributed greatly to the errors made by these acutely brain dysfunctional patients.
Even in this acutely brain dysfunctional group, however, most patients were able to perceive happy and angry faces, much like normal controls. The perception of happy and angry faces may be so crucial to survival that even in acutely impaired patients this ability is relatively preserved until overall cognitive status is reduced massively. What these acutely brain dysfunctional patients do demonstrate, however, is a pattern similar to that shown by normal individuals. They have the most difficulty in interpreting an ambiguous face—the one showing fear or surprise.
Perception of Facial Affect and Right Hemisphere Lesions
Although the perception of facial emotion can be disturbed after various forms of brain injury, a number of studies have suggested that patients with right hemisphere lesions may be especially prone to such errors. Right hemisphere lesions are often associated with impairments in the ability to express emotion and to observe facial expressions, and the prosody of free speech can be reduced.[4,9] Mandal and coworkers [13] reviewed supporting evidence from Wechsler,[22] Heilman et al.,[11] Bihrle et al.,[2] Borod et al.,[3] and Bowers et al.[4] and found that individuals with right hemisphere damage demonstrate impairments in memory for emotional material, the comprehension of emotional tone, the appreciation of humor, and the perception or judgment of emotional faces.
These studies, however, have often been conducted on postacute patients. Patients with acute injuries, even those diagnosed with a localized or unilateral injury, may suffer from bilateral cerebral dysfunction as a result of diaschisis.[16,21] Their pattern of perception may therefore be different.
In our study of 100 acutely impaired brain dysfunctional patients, 40% demonstrated difficulties in perceiving facial affect accurately. In contrast, only 3% of the age-, education-, and gender-matched controls demonstrated this difficulty. Failure to perceive facial affect was not related to demographic variables, lesion location, or diagnosis in this acute population. Thus, the often reported role of the right hemisphere in producing errors in the perception of facial affect was not observed in this acute population.
A possible explanation for this finding is that patients with acute injuries may show bilateral cerebral dysfunction. Studies have demonstrated that during the acute phase of injury unilateral lesions may produce bilateral behavioral abnormalities.[20] Evidence for diaschisis has also been found in studies using positron emission tomography and single photon emission computed tomography.[16] Patients in our study were a mean of 17 days postinjury. In contrast, many studies relating perception of facial affect to brain injury have involved patients a minimum of 1 month to a maximum of 7 years beyond injury (mean, 2.3 years). Thus, the specific effect on the right hemisphere could not be determined in our patient group.
Implications of Qualitative Errors
The nature of the patients’ qualitative errors is important to understanding this difficulty. Almost 70% of the errors made by acutely brain dysfunctional patients reflected either a pejorative response or one of uncertainty. This pattern is interesting given the theoretical work of Weinstein and Kahn.[24] Weinstein[23] has argued that brain dysfunctional patients who show anosognosia for hemiplegia often describe the hemiparetic arm with pejorative terminology. For example, some of his patients described a hemiparetic arm as a “piece of rusty machinery” or “dead wood.” Patients, however, may also respond in a manner that suggests a confusional state. In this study, many patients described the fearful or surprised face as “confused” or “uncertain.” Such responses may reflect the patient’s phenomenological state. When confronted with an ambiguous facial emotion, patients may project their internal state onto that stimulus.[1]
Understanding patients’ confusion and frustration is crucial for rehabilitation planning.[18,21] The present data suggest that brain dysfunctional patients may experience the world in a more confused fashion than they subjectively report or that may be gleaned from their neuropsychological test findings. Weinstein[23] has argued that patients’ choice of words to describe their condition may give rich insights about their phenomenological state and whatever impairment in awareness they may have.
We support this position and suggest that clinicians need to attend to patients’ choice of words when they fail to perform a given task. Their word choice may not only reveal the nature of their cognitive deficit but their personal reaction to the deficit. This is an important source of information often excluded from formal assessments of higher cerebral functioning (including the assessment of facial affect). Such assessments, however, should include both patients’ phenomenological state and their actual performance on neuropsychological testing. This information is helpful for rehabilitation planning.
Whether patients’ qualitative errors can be viewed as a true projective response needs to be considered seriously. Mandal and Bhattacharya [14] argue that errors in the perception of facial affect involve patients’ emotional state. When normal individuals are shown photographs of different facial affects, their errors seem to be spread randomly across different categories of affect. In contrast, depressed subjects make significantly more errors when the affect involves sadness. Furthermore, patients described as anxiety neurotics made significantly more errors when perceiving fearful faces. Women classified as depressed more often identify neutral faces as angry than nondepressed patients.[6] Given the psychoanalytic notion that anger is often related to depression, patients’ primary emotional state may well dictate how they perceive ambiguous stimuli.
Conclusion
When patients perceive a face as confused rather than as fearful or surprised, their response may mirror their internal experience. Brain dysfunctional patients often show signs of perplexity in their responses to the ambiguous stimuli of the Rorschach.[1,17] Patient’s responses often reflect internal emotional or cognitive states that they could not easily articulate. Overall, projective testing in neuropsychological assessment has been abandoned. However, we believe that the use of perception of facial stimuli, which represent different affective states, may be an important part of the clinical neuropsychological examination for both neuropsychological and clinical psychological reasons. When therapists better understand what patients experience, they can better engage patients in rehabilitation activities that help reduce confusion and frustration.[18]
*A T-score is a standardized score in which the mean is artificially set at 50 with a standard deviation (SD) of 10. Patients who have a T-score of 35 or less are 1.5 SDs or more below the mean.
References
- Baker G: Diagnosis of organic brain damage in the adult, in Klopfer B (ed): Developments in the Rorschach Techniques. New York: World Book Company, 1955, pp 319-375
- Bihrle AM, Brownell HH, Powelson JA, et al: Comprehension of humorous and nonhumorous materials by left and right brain-damaged patients. Brain Cogn 5:399-411, 1986
- Borod JC, Koff E, Perlman Lorch M, et al: The expression and perception of facial emotion in brain-damaged patients. Neuropsychologia 24:169-180, 1986
- Bowers D, Bauer RM, Coslett HB, et al: Processing of faces by patients with unilateral hemisphere lesions. I. Dissociation between judgments of facial affect and facial identity. Brain Cogn 4:258-272, 1985
- Broks P, Young AW, Maratos EJ, et al: Face processing impairments after encephalitis: Amygdala damage and recognition of fear. Neuropsychologia 36:57-70, 1998
- Crews WD, Jr., Harrison DW: Cerebral asymmetry in facial affect perception by women: Neuropsychological effects of depressed mood. Percept Mot Skills 79:1667-1679, 1994
- Darwin C: The Expression of the Emotions in Man and Animals, 3rd ed. New York: Oxford University, 1998
- Ekman P, Friesen W: Unmasking the Face: A Guide of Recognizing Emotions from Facial Clues. New Jersey: Prentice Hall, 1975
- Gardner H, Ling PK, Flamm L, et al: Comprehension and appreciation of humorous material following brain damage. Brain 98:399-412, 1975
- Heilman K, Valenstein E: Clinical Neuropsychology. New York: Oxford University, 1993
- Heilman KM, Bowers D, Speedie L, et al: Comprehension of affective and nonaffective prosody. Neurology 34:917-921, 1984
- Kurucz J, Feldmar G: Prosopo-affective agnosia as a symptom of cerebral organic disease. J Am Geriatr Soc 27:225-230, 1979
- Mandal MK, Asthana HS, Pandey R, et al: Cerebral laterality in affect and affective illness: A review. J Psychol 130:447-459, 1996
- Mandal MK, Bhattacharya BB: Recognition of facial affect in depression. Percept Mot Skills 61:13-14, 1985
- Panksepp J: Affective Neuroscience. The Foundations of Human and Animal Emotions. New York: Oxford University, 1998
- Perani D, Vallar G, Paulesu E, et al: Left and right hemisphere contribution to recovery from neglect after right hemisphere damage-an [18F] FDG PET study of two cases. Neuropsychologia 31:115-125, 1993
- Piotrowski ZA: The Rorschach ink blot method in organic disturbances of the central nervous system. J Nerv Ment Dis 85:525-537, 1937
- Prigatano GP: Principles of Neuropsychological Rehabilitation. New York: Oxford University, 1999
- Prigatano GP, Amin K, Rosenstein L: Administration and Scoring Manual for the BNI Screen for Higher Cerebral Functions. Phoenix, AZ: Barrow Neurological Institute, 1995
- Prigatano GP, Wong JL: Speed of finger tapping and goal attainment after unilateral cerebral vascular accident. Arch Phys Med Rehabil 78:847-852, 1997
- Prigatano GP, Wong JL: Cognitive and affective improvement in brain dysfunctional patients who achieve inpatient rehabilitation goals. Arch Phys Med Rehabil 80:77-84, 1999
- Wechsler AF: The effect of organic brain disease on recall of emotionally charged versus neutral narrative texts. Neurology 23:130-135, 1973
- Weinstein E: Anosognosia and denial of illness, in Prigatano GP, Schacter DL (eds): Awareness of Deficit After Brain Injury. New York: Oxford University, 1991, pp 240-257
- Weinstein E, Kahn R: Denial of Illness: Symbolic and Physiological Aspects. Springfield, IL: Charles C Thomas, 1955