
Targeted Tissue Engineering in Spinal Surgery. The Bone Morphogenetic Proteins: Past, Present, and Future
Authors
Christopher P. Ames, MD*
Eric W. Nottmeier, MD**
Curtis A. Dickman, MD
Volker K. H. Sonntag, MD
Division of Neurological Surgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Current Addresses:
*Department of Neurosurgery, UCSF, San Francisco, California
**Department of Neurosurgery, Mayo Clinic, Jacksonville, Florida
Abstract
In the next decade, advances in molecular biology and tissue engineering will affect all areas of neurosurgery. Spinal surgery in particular will benefit from the use of recombinant bone morphogenetic proteins (BMPs) to increase the rates of spinal fusion. This review details the history of the development of the BMPs, focusing on key studies that are helping to move these agents from the laboratory to initial clinical utilization. The molecular biology of these agents is reviewed, and how these agents will affect future developments in spinal materials and outcomes research is discussed.
Key Words: bone morphogenetic proteins (BMPs), iliac crest, ligament repair, spinal fusion, tissue engineering.
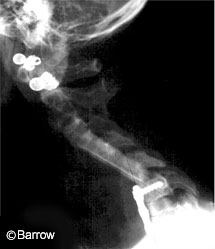
In the last decade, new developments in spinal instrumentation technology have affected surgical techniques in all regions of the spine. Effective instrumentation strategies now offer sufficient rigidity to promote fusion and to achieve multiplanar correction from the occipitocervical junction through the sacrum and pelvis. Nonetheless, the rate of spinal fusion is not yet 100% for any spinal region despite rigid fixation, particularly when multilevel operations are performed.[6] Given that more than 200,000 fusion procedures are performed each year in the United States alone, even a 5% failure rate represents an undesired result in more than 10,000 patients.
A portion of these failures represents failures of instrumentation strategy (e.g., anterior or posterior stabilization alone when a 360 degree strategy is required). Some failures may result from less than optimum surgical technique. Many failures, however, occur despite acceptable placement of the instrumentation and adequate rigidity of the segments to be fused.
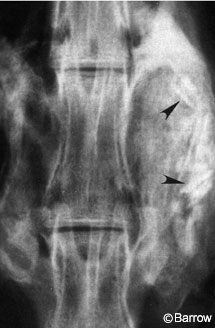
Although immobilization alone creates rigidity through the motion segment, it does not guarantee successful fusion (Fig. 1). Factors that can contribute to such failures include stress shielding, host bone biology, bone graft quality, and the presence of bone growth inhibitory factors such as smoking, nonsteroidal anti-inflammatory drug use, and previous radiation or chemotherapeutic treatment.
To improve the rate of spinal fusion to 100%, strategies are needed to address each of these factors. For the cervical spine, new dynamic plates have been developed to maintain rigidity through the fusion site while allowing vertical subsidence. Thus stress shielding is decreased, and the graft can withstand greater compressive loads in a controlled fashion.[11] Greater compressive loads increase the efficiency of bone formation; nonetheless, poor host bone biology can significantly impede the efficiency and rate of fusion.
It is unlikely that increasing rigidity or increasing load transfer to the graft material will overcome intrinsic host factors that impair the efficiency of fusion. However, the forthcoming introduction of bone morphogenetic proteins (BMPs) to the armamentarium of spinal fusion will likely overcome many of the effects of poor host bone biology and other inhibitory factors.
Our institution has participated in two clinical studies on the use of BMP to enhance spinal fusion. A research project is underway to determine if BMP can reverse the adverse effects of radiation on spinal fusion. This article traces the history of the discovery of BMP, briefly reviews the molecular biology of BMP and its role in bone and cartilage induction, highlights the most important recent work in the field, and attempts to define the role that BMP will most likely play in the next few years.
Discovery of BMP
Marshall Urist[57] is credited with the discovery that demineralized bone matrix proteins can induce the formation of new ectopic bone. In 1965 Urist demonstrated that acid-decalcified long bone from rabbits implanted into abdominal pouches resulted in the ectopic formation of bone. When admixed with the demineralized bone matrix, protein denaturing and blocking agents eliminated this new bone formation. The agents used to decalcify the bone also lysed any osteoblasts or osteoprogenitor cells. Urist therefore concluded that the proteins in this matrix were able to induce osteogenesis and mineralization at ectopic sites, most likely through the recruitment and differentiation of local host cells. Urist stated that “the process is followed immediately by new-bone formation by autoinduction in which both the inductor cells and the induced cells are derived from ingrowing cells of the host bed.” In this original study, Urist also implanted demineralized bone matrix into long bone defects in various small animals, into the lumbar transverse process region in three dogs, and into bone defects in 21 humans. Bone induction was 50% in the dog model despite the significant motion associated with tail wagging. Ninety percent of the human cases demonstrated new bone formation in various bone defects.
Although Urist first demonstrated the existence of these osteoinductive protein elements in demineralized matrix, he was not the first to use decalcified bone to heal bone defects nor was he the first to postulate the existence of osteoinductive factors in bone. In fact, Nicolas Senn of Rush University Medical College[53] first used decalcified bone matrix to treat osteomyelitis more than a century ago. Then in 1945 Lacroix postulated the existence of an osteogenic factor in bone called osteogenin.[29]
The next step in characterizing these osteoinductive factors was the development of a bioassay for osteoinductive activity. The demineralized bone matrix contained numerous proteins, including structural proteins such as type I collagen. Before the protein or group of proteins with the most potent osteoinductive activity could be isolated, purified, and sequenced, a standardized assay for this activity was needed. Interestingly, neither the group of proteins in the solubilized residue nor the group of insoluble proteins in the collagen residue was capable of inducing bone formation alone. Only the combination of these two fractions induced bone formation at ectopic sites. Therefore, the assay, as designed by Sampath and Reddi[47] in 1983, consisted of adding the test protein or purified protein extract to a standard insoluble collagen fraction and implanting this mixture into ectopic sites in rats.
Using the previously developed bioassay for osteogenic activity, the first BMP was purified and partially sequenced in 1989.[33] Initially, this BMP was termed osteogenin but was renamed BMP-3. Based on this partial sequencing, molecular probes were designed to allow the identification and cloning of other BMP family members, including BMP-2, which will be the first of these proteins to be used clinically in the next few months.12,60 BMP-7 and BMP-8 were cloned in 1990 by Ozkaynak et al.[43] BMP-7, also known as OP-1, will most likely be the second BMP to receive widespread clinical use in the next decade.
Molecular Biology of BMPs
The BMPs, members of the transforming growth factor-(beta) (TGF-(beta)) superfamily, are dimeric molecules bound by a single disulfide bond. Initially, they are synthesized as more than 400 amino acid precursors, which are then cleaved at the carboxyl terminal regions to 120 to 140 amino acids of the active molecules. Subtilisin-like proteases are involved in processing the BMP precursor proteins, and BMP activity may be partially regulated at the precursor processing level.[1,14]
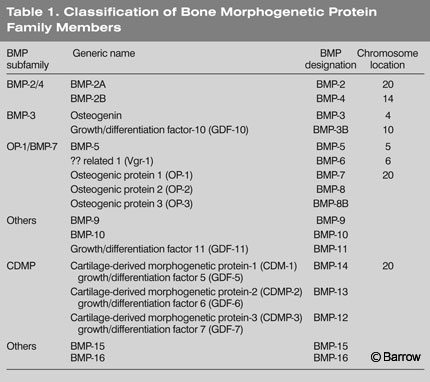
Mature BMPs are released into the extracellular environment where they interact with cell-surface receptors on various cell types (Table 1). The active dimeric molecules consist of both homodimers and heterodimers. The latter are dimers formed from two different BMPs such as BMP-2 and BMP-4. Several heterodimers are more potent than homodimers. For example, BMP-2/BMP-7 and BMP-2/BMP-6 are 5 to 10 times more potent in inducing cartilage and bone formation than BMP-2 homodimers.[28] Furthermore, a receptor with a specific affinity for a BMP-2/BMP-4 heterodimer has been identified.[37] The x-ray crystallographic structure of BMP-7 demonstrates the characteristic cysteine knot of BMP family members.[22]
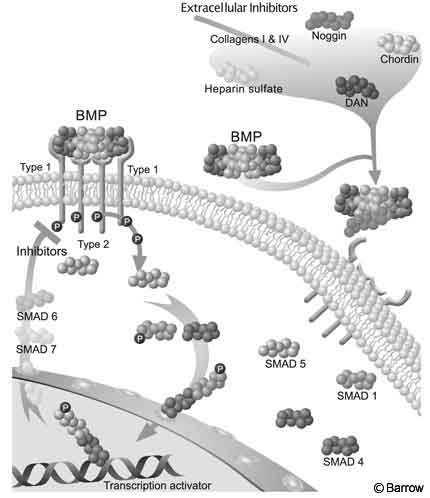
Like members of the TGF-(beta) superfamily, BMPs act through ligand-induced hetero-oligomerization of Type I and Type II receptors. These receptors function as serine/threonine protein kinases. The signaling cascade begins when the BMP dimer finds the extracellular domain of a Type II receptor, which is constitutively active. Next, a Type I receptor binds to the BMP and is phosphorylated by the activated Type II receptor. This activated Type I receptor then phosphorylates members of the Smad family, which function as the intracellular second messengers in the BMP signaling cascade. Smad proteins are activated in a ligand-specific fashion.
Eight different types of Smad proteins have been identified. Smad 1 and Smad 5 are activated by BMP-2 and BMP-4. Next, phosphorylated Smad 1 and Smad 5 form heteromeric complexes with Smad and are then translocated into the nucleus where they function as transcriptional activators.[16,17] Smad 6 and Smad 7 appear to inhibit the phosphorylation and thus activation of Smad 1 and Smad 5 and may provide a negative regulatory function to the BMP signaling cascade (Fig. 2).[46]
Five Type II receptors and seven Type I receptors have been identified in mammals.[38] Each BMP binds with various affinities to the various Type I and Type II receptors. The affinity for the Type I receptor is thought to be the largest determinant of the final specific biological effect. Certain BMPs can bind to numerous Type 1 receptors thus producing multiple different responses in various cell and tissue types. The final biological response to BMP thus depends not only on the type and concentration of BMP present but also on the type and concentration of BMP Type 1 receptors and inhibitory signals such as Smad 6 and Smad 7.
Additional BMP inhibitors include the Noggin, Chordin, and DAN proteins, which are found in the extracellular matrix. They bind with high affinity to BMPs, thus preventing the latter from binding to Type II receptors. The constituents of the extracellular matrix, including collagen Type I and IV and heparin sulfate, also bind to the BMPs, thus regulating their bioavailability. Regulation of the BMP pathway may occur at any interaction along the cascade, including inactivation of SMAD proteins, nuclear translocation, and deoxyribonucleic acid (DNA) binding.
BMP Induction of Bone Formation
BMPs initiate an osteoinductive cascade that terminates in the formation of new bone. BMPs also appear to play an active role in each stage of this process once it has begun. Osteogenesis can occur via two distinct pathways: endochondral and intramembranous. Bone morphogenesis by the endochondral pathway involves a complex cascade with at least three distinct phases: chemotaxis and mitosis of mesenchymal cells, differentiation of mesenchymal cells into cartilage, and replacement of cartilage by bone. The intramembranous pathway consists of two stages: chemotaxis and mitosis of mesenchymal cells and differentiation of the mesenchymal cells directly into bone. Most histological studies performed on BMP-induced osteogenesis have found some evidence of an intermediate stage of cartilage formation.[46,59] The mechanism of BMP-induced osteogenesis is somewhat region dependent, however, with the intramembranous pathway occurring in the regeneration of bone of the facial skeleton.
The BMPs, particularly -2, -4, -5, -7 and -14, play a significant role during embryonic development of the skeleton. The exact mechanism and role remain poorly characterized because BMP-2 and BMP-4 gene knockouts are lethal to embryos. BMP-7 knockouts are characterized by significant abnormalities of skeletal patterning. The production of bone in ectopic sites in adult animals, including humans, is thought to involve a partial recapitulation of similar mechanisms involved in the embryonic formation of the skeleton. For example, BMP-2 implanted into a rabbit knee defect does not cause complete bone formation along the articular surface. Instead, bone is produced below the articular surface, and cartilage is regenerated along the cartilaginous joint surface. Obviously, the presence of BMP-2 produces novel osteogenesis and chondrogenesis, which respond to external regulatory and location-dependent signals that generate appropriate tissue types in particular locations.[51,52]
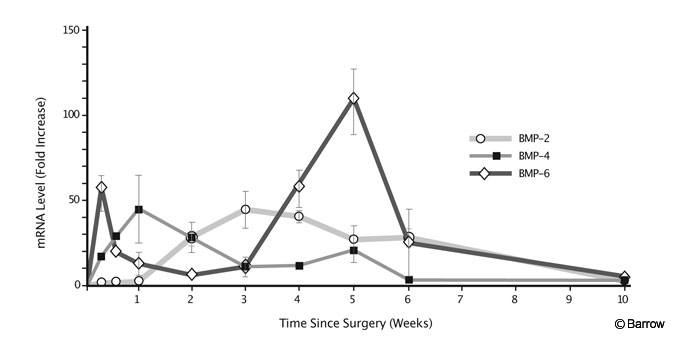
During the process of new bone formation, the BMPs are expressed in a specific temporal pattern (Fig. 3). The time course of expression has been best characterized in the rabbit intertransverse fusion model.[40] BMP-6 levels first peak at Day 2 and then again at Week 5. BMP-4 peaks at Week 1 and BMP-2 mRNA (messenger ribonucleic acid) levels reach a maximum at Week 3. This considerable overlap suggests a codependency and perhaps redundancy of function. Specific BMPs also stimulate and inhibit each other. In a study using fetal rat calvarial cells, BMP-2 increased the levels of BMP-3 and BMP-4 mRNA.[13] In an osteosarcoma cell line, BMP-7 stimulated BMP-6 mRNA while decreasing levels of BMP-2 and BMP-4.[27]
Therapeutic Applications in Spinal Surgery
Although no BMP has yet been approved for spinal fusion by the Food and Drug Administration (FDA), considerable research has been performed on delivery systems, dosage requirements, and safety in lower animal models and nonhuman primates. Recently, human preclinical trials have begun. Numerous studies performed over the last decade have demonstrated the clinical efficacy and safety of the BMPs for inducing spinal arthrodesis. The levels of active BMPs in demineralized matrix, however, depend on extraction methods, storage, and processing. Typically only 1 to 2 mg of BMP are found in 1kg of cortical bone.[32]
Although demineralized bone matrix contains a mixture of BMPs, which, theoretically, may be superior to a single BMP and effectively promotes spinal fusion,[32] recent work suggests that it functions best as a graft extender at ratios as high as 3:1. It does not increase fusion rates when added to standard quantities of autograft. When less than standard quantities of allograft are used, however, the addition of demineralized bone matrix can restore fusion rates to levels comparable to those associated with standard quantities of allograft.[39] Newer, fiber-based sheet and putty formulations of demineralized bone matrix may be more effective than the older particle-based types.[35]
Much of the early work with purified, concentrated BMP extracts and recombinant BMPs used critical-sized bone defect models. Critical-sized bone defects are defects that will not heal naturally without grafting. The area involved varies with the species and particular bone studied (e.g., rat femur, rabbit fibula). In 1992 Yasko et al.[61] used a rat segmental femoral defect model and found that rhBMP-2 combined with bone marrow can produce union rates of 100%. This result was three time higher than that achieved with autologous cancellous bone graft. BMP-2 has also been used successfully to repair mandibular defects, demonstrating its ability to form bone by the intramembranous pathway.[41,56] Muschler et al.[41] evaluated the effectiveness of rhBMP-2 in a biodegradable polylactate polyglycolate carrier in a dog intertransverse fusion model. Union rates and biomechanical strength were equivalent in both the autograft and PLGA/BMP-2 groups.
In 1994 Cook et al.[15] demonstrated the ability of rhOP-1 (rhBMP-7) applied on a collagen carrier to induce spinal fusion in the posterolateral region in a dog model. The BMP-treated group developed a solid fusion 12 weeks after surgery compared with 26 weeks for the autograft control group. This study was the first to demonstrate that a BMP was not merely a viable alternative to allograft but was truly superior in its ability to produce efficient fusion.
In 1995 Schimandle et al.[50] again demonstrated this concept by using the rabbit posterolateral fusion model developed by Boden et al.[9] In this study, the rhBMP-2 experimental animals achieved a more biomechanically rigid fusion in less time than control autograft animals (Fig. 4). These fusions were biomechanically stronger and stiffer than autograft controls. The fusion rate of the BMP animals was 100% at 5 weeks compared to only 42% of the control autograft fusions. The Boden rabbit model is particularly good because of its reproducibility and its baseline nonunion rate of about 35%, which approximates that of human posterolateral fusion.
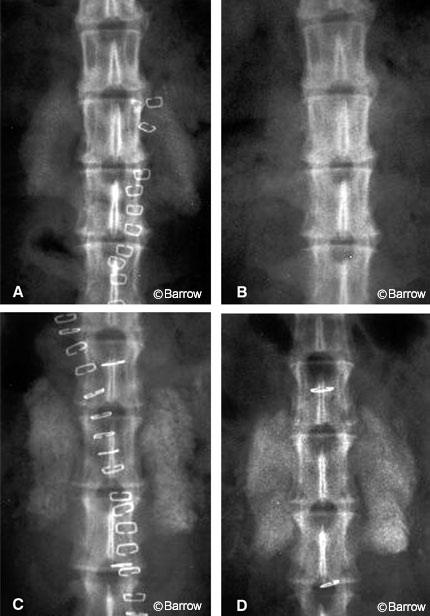
In 1996 Holliger et al.[26] evaluated the morphology of BMP-2 induced fusion in the rabbit intertransverse model with computed tomography. The BMP-2 fusions involved significantly greater bone volume and formed better attachments to the adjacent spinous processes than autograft. This finding may explain the greater biomechanical rigidity of these fusions. In the autograft controls, the weak point of the fusion was usually at the attachment to the transverse processes. In the BMP animals, however, the weak point was randomly distributed throughout the fusion mass.
In the last 5 years, considerable research has focused on optimization of the BMP dose and carrier. The BMP carrier regulates the pharmacokinetics of delivery, possibly as an osteoconductive scaffold, and certainly as a key determinant of the local fusion microenvironment. The carrier affects the optimum implant dosage.[44] Sandhu et al.[49] studied the effect of escalating rhBMP-2 dose in an adult beagle intertransverse fusion model using an open cell polylactate polymer carrier compared to autograft. All BMP-2 specimens were solidly fused by 3 months. There were no significant differences in biomechanical, radiographic, or histologic characteristics over a 40-fold dose escalation from 58 mg to 2300 mg.[48]
In 1999 Martin et al.[35] described their experience with various doses and carriers in nonhuman primates. Their landmark study showed that the dosage and carrier data accumulated from small animals models cannot be generalized to determine BMP protocols in primates and humans. With the same collagen carrier used in rabbit and dog studies, a minimum dose of 18 mg was required to obtain a fusion rate higher than 50% in monkeys. Even a 32-mg dose did not work consistently (only 50% fused). If a polylactic acid (PLA) carrier was used, however, only a 4-mg dose was required to obtain a 100% fusion rate. The authors postulated that the failure rate of the collagen carrier was largely caused by overly rapid delivery of the BMP from muscular pressure on the deformable collagen sponge. When the sponge was “protected” from compression by a nondeformable porous mesh, the fusion rate was 100% with a 9-mg dose.
Nonhuman primates and humans appear to require significantly higher doses of BMP than lower animal models and may require different carriers for certain indications. Recent work suggests that it may be possible to provide an osteoconductive carrier that is also compression resistant. Boden et al.[8] studied the effect of dose escalation in a nonhuman primate model of intertransverse fusion using a 60% hydroxyapatite and 40% tricalcium phosphate carrier block. All BMP-2 treated animals achieved spinal fusion (dose, 0, 6 mg, 9 mg, 12 mg per side), and increasing the dose increased the amount and quality of bone formed. Despite such successful fusion, the ceramic material resorbs very slowly. Even at 24 weeks a substantial portion of ceramic remained outside the fusion mass hindering radiographic detection of novel bone formation.
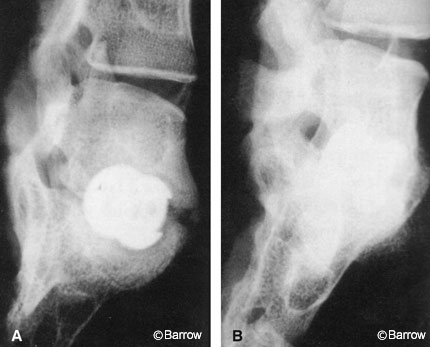
The 60/40 carrier, which resembles coarse sand, also has poor handling characteristics. Consequently, other hydroxyapatite tricalcium phosphate carriers with slightly lower density and better handling characteristics have been evaluated. Recently, both a 95:5 tricalcium phosphate hydroxyapatite-impregnated collagen sponge carrier and an 85:15 collagen sponge were evaluated in a nonhuman primate model at two dose concentrations (BMP/gm of carrier).[55] Because of their compression resistance, these carriers are referred to as compression-resistant matrices (CRMs). The 85:15 material proved to be more effective than the 95:5 material in resisting compression and thus forming a larger fusion mass. Both materials were effective in producing fusion when the total implant dose of 9 mg was distributed over two CRMs (100% at 24 weeks). In addition, the fusion was better visualized radiographically compared to the 60:40 material (Fig. 5).
This study[55] first documented the effect of BMP concentration. Prior studies had focused primarily on total dose. A total dose of 9 mg was used in all cases for the primate model. When this dose was distributed over four CRM sponges rather than two, resulting in a concentration of 1.07 mg/ml compared with 2.14 mg/ml, radiographic fusion was not achieved. Human clinical trials to evaluate the efficacy of BMP in a CRM carrier for posterolateral fusion will begin within the next year at our center and at others.
The effectiveness of rhBMP-2 in producing anterior interbody fusion has also been evaluated in lower animals and primates. Boden et al.[7] demonstrated that rhBMP-2 in a collagen sponge carrier produced effective fusion when inserted laparoscopically in a titanium threaded cage into the lumbar spine of monkeys (Fig. 6). The collagen carrier has proven equally effective when used inside an impacted allograft in a primate anterior lumbar interbody fusion (ALIF) model.[23] At the lumbosacral junction (L7-S1), three monkeys treated with freeze-dried allograft and rhBMP-2 achieved radiographic signs of fusion as early as 8 weeks. Control autograft animals were much slower to develop fusion and two failed to fuse.
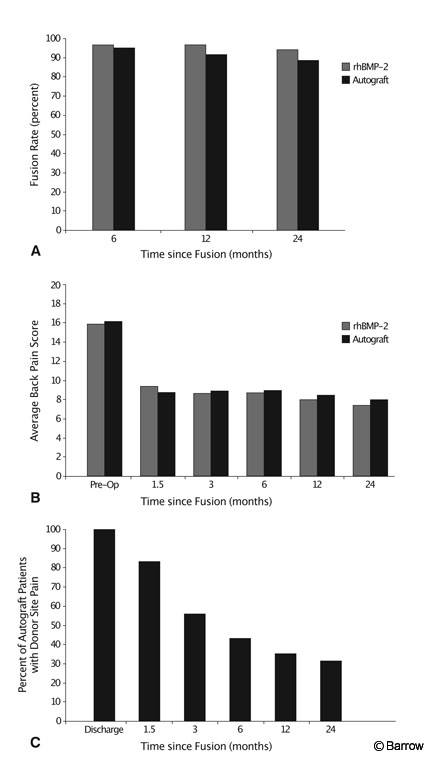
In 2001 Dickman et al.[18] reported the first randomized prospective trial of the clinical use of rhBMP-2 for ALIF as an alternative to autograft in humans. Overall, 281 patients were enrolled (145 investigational and 136 control) at 16 centers. All patients had failed at least 6 months of conservative therapy with single-level degenerative disk disease resulting in intractable lower back or radicular pain. All patients underwent single-level ALIF procedure with a tapered lordotic threaded cage placed through an open approach. Standard outcome measures were used. The rhBMP-2 reduced operative time (1.7 vs. 2 hours) and blood loss (109.3 vs. 153.8 cc) and increased fusion rates (96.0% vs. 92.5%). Obviously, all rhBMP-2 patients were spared donor site pain (Fig. 7). No adverse outcomes associated with the use of rhBMP-2 were reported.
Given the success of rhBMP-2, it is not surprising that its use in posterior lumbar interbody fusion (PLIF) has also been reported.[3] A prospective randomized study of 71 patients compared PLIF with a threaded interfix cage containing rhBMP-2 on the standard collagen carrier to iliac crest autograft. BMP-2 produced comparable results to autograft in terms of perioperative course, fusion rate, and functional pain/outcome scores at a 12-month follow-up. However, 40% of rhBMP-2 patients had moderate or appreciable bone formation in the ventral epidural space posterior to the cages. The amount of bone did not correlate with any clinically important outcome parameter. The bone formation was thought to have most likely resulted from leakage of the BMP-2 from the carrier into the interbody surgical site and the normal postoperative blood clot that may form after the fusion site is prepared. In the ALIF procedure, the posterior longitudinal ligament is left intact, and it serves as a natural barrier. New carriers with slower release kinetics, such as a form of CRM or perhaps a resorbable barrier sheeting, could be used to decrease the incidence of this complication. Furthermore, implants such as allograft wedges instead of cages may contain the BMP inside the graft in contrast to the porous threaded cages. For the immediate future, it is likely that the use of BMP for interbody procedures will be restricted to ALIF.
BMP-2 has also been evaluated in animal and preliminary human studies for use in the cervical spine. Zdeblick et al.[63] used rhBMP-2 in a threaded cervical cage in a goat multilevel diskectomy model. Control (autograft) animals achieved a 47% fusion rate compared with a 95% fusion rate in those receiving rhBMP-2. A prospective multicenter human clinical trial of BMP-2/collagen sponge in allograft for single- and two-level anterior cervical diskectomy has also been studied in 33 patients.[5] The BMP-2/collagen sponge was placed within a machined allograft (Cornerstone SR) and compared with iliac crest autograft also placed within a machined allograft. A cervical plate was placed in all cases. The fusion rate was 100% in both groups, and no adverse effects were reported. One BMP-2 patient, however, experienced ossification at one disk level adjacent to the fusion. Compared to controls, patients in the rhBMP-2 group reported improved patient satisfaction and perceived effect scores at 1 year. Although this review has focused on BMP-2 because it is the best characterized of the BMPs and the one most likely to receive FDA approval first, other BMPs, particularly BMP-7 (OP-1), have also been proven effective for similar indications in other animal studies.[21]
In every case rhBMP-2 has proven to be at least as effective as autograft in producing fusion, and it spares patients donor site pain and related complications. In most cases and at most locations, rhBMP-2 is more effective than autograft and produces faster and more rigid fusions. One of its most effective uses, however, may be for patients who have an inherently compromised capacity for bone healing: smokers; patients who require postoperative therapy with nonsteroidal anti-inflammatory drugs (NSAIDs) or steroids; patients whose potential fusion site has been compromised by previous or current radiation therapy; and patients with diabetes, osteoporosis, metabolic bone diseases, and disorders of calcium metabolism.[36] OP-1 (BMP-7) has reversed the adverse effect of nicotine on spinal fusion in a rabbit model. A 100% fusion rate was achieved in rabbits receiving OP-1 compared to a 25% fusion rate in the rabbits receiving autograft.[45]Furthermore, BMP-2 has overcome the inhibitory effects of NSAIDs on fusion in 100% of the animals (rabbits) that received ketorolac and BMP-2 compared to 35% for the group that received ketorolac and an autograft.[34] Whether BMP-2 can overcome the adverse effects of radiation on spinal fusion is under investigation at our institution. If the BMPs can initiate osteogenesis at a radiated site, it may be possible to use allograft bone instead of methylmethacrylate to reconstruct the spine of patients with malignant tumors.
Traditionally, the BMPs are thought of as growth factors. Actually, however, they act as differentiation factors both during development and when implanted in adult animals. Initial concern that they would act as tissue mitogens causing growth of neoplastic tissue has not been confirmed. In fact, just the opposite seems to be the case. Orui et al.[42] studied the effect of rhBMP-2 on cell lines of osteosarcoma, prostate cancer, and breast cancer. In these cell lines, rhBMP-2 promoted no growth, inhibited growth of two of the osteosarcoma lines tested, and induced differentiation of the osteosarcoma cell lines to a more mature phenotype. Similar results have been reported for breast, multiple myeloma, and thyroid cancer cell lines.[19,20,25] More research, including the analysis of BMP receptor expression in these cell lines, is needed to fully characterize the effect of BMP on common types of primary and metastatic spinal neoplasms.
Gene Therapy as a BMP- Delivery Mechanism
Although the collagen carrier works well for most interbody indications and the CRM formulations are promising for use in the posterolateral region, both delivery vehicles require a significant volume of carrier to deliver BMP. They are also both subject to possible failure from protein degradation and poor incorporation of the protein into the carrier. Gene therapy has therefore been evaluated as a possible delivery mechanism for BMP implantation. Although gene therapy for systemic diseases has been largely unsuccessful for this particular indication, long-term expression would be unnecessary (and perhaps undesirable). In fact, only transient expression of levels of protein sufficient to induce the osteogenic cascade would be required.
Gene therapy approaches using both ex vivo and in vivo methods have been tested. In the ex vivo method, transfection of marrow cells or mesenchymal cells with the cDNA (e.g., adenovirus hBMP-2 cDNA) occurs outside the body. The transfected cells are then implanted. This approach has been used successfully to form ectopic bone in leg muscles in rats and to heal femoral defects.[30,31] The in vivo gene therapy technique (direct injection of the transfection vector) has also been used successfully. Direct injection of an adenoviral vector containing BMP-2 cDNA into the posterior spine of nude rats has induced bone formation.[2,62]
The gene therapy approach is also optimal for the delivery of intracellular nonsecreted factors that may play key roles in the induction of osteogenesis. For example, using differential display polymerase chain reaction, a protein known as LIM mineralization protein-1 was discovered in 1998. This technique compares RNA expression profiles from stimulated and unstimulated osteoblast cultures to identify factors that may be involved in the differentiation of osteoblasts. In situ hybridization studies from mouse embryos have shown that LMP-1 expression coincides spatially and temporally with the transition from hypertrophic cartilage to bone trabeculae during endochondral ossification. The intracellular expression of LMP-1 may stimulate the cell to produce or release secreted osteoinductive factors, including BMPs, which then recruit neighboring cells into the osteoinductive cascade. In 1998 Boden et al.[10] used ex vivo gene therapy techniques to produce LMP-1 transfected marrow cells, which they implanted in the posterior spinal region of athymic mice with demineralized bone matrix. Fusion was achieved in 100% of the animals. All rats that received marrow cells transfected with a reverse copy of LMP-1 failed to fuse.
Gene therapy offers the advantage of minimally invasive delivery, smaller requisite volumes, and sustained protein presence. Furthermore, it allows the possibility of targeting expression to particular cell types through tissue-specific promoters (transcriptional targeting) or through modified vectors with tissue-specific tropism (transductional targeting). It also may be possible to modulate expression temporally by using inducible promoters that can turn on in response to the presence of a pharmacological agent such as tetracycline. Such cellular and temporal targeting may make it possible to design systemic strategies to combat metabolic bone diseases such as osteoporosis.
The disadvantages of gene therapy approaches include the antigenicity of the viral vector, regulation of the amount of bone production, poor efficiency of successful transfection (usually less than 50%), and extremely high costs.[24]
Cost-Benefit Analysis of rhBMP-2

bone graft.
In today’s highly regulated arena of health care costs and declining reimbursements, the cost-benefit ratio for any new technology or product must be evaluated. Gene therapy delivery methods would be extremely expensive per patient and may be impractical for routine spinal fusion surgery. Even standard delivery methods would necessarily reflect the development, production, and purification costs of a recombinant protein, which understandably would be substantial. To determine whether the widespread use of rhBMP-2 for spinal fusion is practical from an economic perspective, comparing the cost of the protein to the cost of allograft or autologous bone graft harvesting is inadequate. Each indication, including increase (if any) in fusion rate, pseudarthrosis repair costs, operating room time, length of hospital stay, and the need for additional instrumentation, must be examined. All of these criteria may need to be stratified further by bone quality and the presence of bone-healing inhibitors such as NSAIDs, smoking, and radiation.
Shaffrey et al.[54] recently presented a cost analysis from an institutional and payers’ perspective. They examined many of these criteria for a single-level threaded cage ALIF. The fusion rate achieved with rhBMP-2 was 97% compared to 92% for iliac crest autograft. From the payers’ perspective, $359 was saved per patient. From a hospital perspective, 60% of the costs of rhBMP-2 were offset. When the parameters of this model were varied to reflect a “more typical” patient population, the cost savings increased to $3000/patient and the entire cost of the growth factor was offset.
The Immediate Future for BMPs
The FDA will likely approve rhBMP-2 for ALIF. The approval will be specific for the use of rhBMP-2 on a collagen carrier (Fig. 8, InFuse, Medtronic, Sofamor Danek; Memphis, TN) to be used in the LT cage (Medtronic Sofamor Danek; Memphis, TN). Significant off-label use, including the use of other cages and for other indications beyond standard ALIF, is also likely.
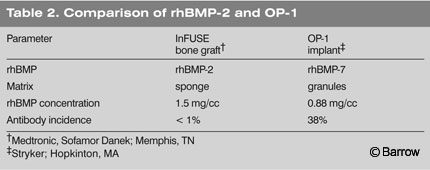
The next BMP product that is likely to be approved is OP-1 (BMP-7) manufactured by Stryker Biotech (Hopkinton, MA; Table 2). It is unclear whether similar BMPs, combinations of BMPs, and carrier variations developed in the future will be eligible for the expedited 510K approval process. Given the slow pace with which the FDA initially approved rhBMP-2, full review will likely be required for all new products at least in the immediate future.
Future Directions in BMP Research
The use of BMPs in surgery falls under the much wider heading of targeted-tissue engineering, which involves the ability to control the production or regeneration of a chosen tissue type in a certain location. In addition to bone formation, ligament regeneration or repair could be targeted in cases of spinal trauma or connective tissue disease or cartilage maintenance and repair in cases of symptomatic intervertebral disk degeneration.
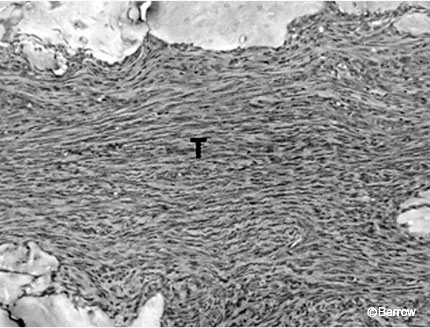
BMP-12, -13, and -14 are also known as cartilage-derived morphogenetic proteins 3, 2, and 1, respectively. They induce tendon and ligament formation when included on collagen carriers implanted into ectopic sites. The implanted sites demonstrated no cartilage or bone formation and were positive for immunohistochemical markers for adult tendon and ligament including elastin (Fig. 9).[58] In addition, BMP-13 and BMP-14 improve the strength of an Achilles’ tendon repair.[4]
BMP-7 (rhOP-1) has also been investigated as a stimulator of cell proliferation, proteoglycan synthesis, and collagen synthesis in cultured rabbit intervertebral disk cells.[20] Disks of New Zealand white rabbits were harvested and cultured, and annular material and nucleus pulposus were separated and cultured. Cultures were incubated with various doses of rhOP-1 for various times, and a significant mitogenic effect was noted. A dose-dependent increase in proteoglycan synthesis was noted in the nucleus pulposus and annulus fibrosis cultures. Likewise, collagen synthesis increased in a dose-dependent manner.
Further work has demonstrated the ability of OP-1 to restore normal levels of sulfated proteoglycans after enzymatic degradation was used to simulate disk degeneration.[30] In fact, prolonged OP-1 treatment actually resulted in supranormal levels of proteoglycan synthesis in the cultured cells. The possible usefulness of OP-1 for degenerated disk repair will be an active area of research in the next several years. To some extent, the action of the growth factor appears to depend on the local environment in which it is placed (e.g., disk cells, cancellous bone, muscle). Even BMPs such as OP-1, which are strongly osteoinductive in certain situations, serve as potent morphogens for other tissue types. In the previously described example, the disk cells and proteoglycan scaffolds were formed instead of bone. Whether the same result would occur in vivo is unclear.
Conclusions

The next decade will witness many changes in spinal surgery—the widespread use of minimally invasive therapies, the introduction of artificial disks to the market in the United States, the increasing use of image guidance, implants made of resorbable polymers, and, perhaps most importantly, the use of targeted-tissue engineering in the form of BMP implantation to almost eliminate pseudarthrosis and the need for iliac crest grafts. How will spinal surgery be affected by the ability to produce what will most likely be an almost 100% fusion rate in all patients? Will fusion become a more popular operation once pseudarthrosis-related complications are eliminated from the variables affecting outcome? How will the various advances be integrated to direct the course of spinal surgery in the future?
Although it is difficult to predict the future in the rapidly changing field of spinal surgery, certain changes are reasonably certain. The use of BMPs will definitely provide an unparalleled opportunity to determine the true clinical failure rate of spinal fusion by eliminating patients with nonunions and those who suffer from chronic pain at the iliac crest donor site from the clinical failure groups. More rapid rates of fusion and earlier biomechanical stiffness of the fusion mass may allow less rigid materials, including resorbable polymer implants, to be used for various indications including anterior cervical plating (Fig. 10). With less need for decortication and the elimination of the need for bone graft implants, percutaneous and minimally invasive fusion techniques will be emphasized. Finally, and perhaps most exciting of all, will be the very real opportunity to avoid fusion procedures altogether (and the stresses that they induce at adjacent levels) through the clinical application of targeted spinal ligament and disk regeneration. We may be witnessing the beginning of the true next generation of spinal reconstruction in which natural spinal stabilizing systems will be rebuilt rather than replaced.
References
- Akamatsu T, Matsuda Y, Tsumura K, et al: Subtilisin-like proprotein convertase PACE4 (SPC4) is a candidate processing enzyme of bone morphogenetic proteins during tooth formation. Dev Dyn 216:481-488, 1999
- Alden TD, Pittman DD, Beres EJ, et al: Percutaneous spinal fusion using bone morphogenetic protein-2 gene therapy. J Neurosurg 90:109-114, 1999
- Alexander JT, Branch CL, Haid RW, et al: An analysis of the use of rhBMP-2 in PLIF constructs: Clinical and radiographic outcomes (abstract). AANS/CNS Joint Section on Spine and Peripheral Nerves, February 2002, Orlando, FL, p 26, 2002
- Aspenberg P, Forslund C: Enhanced tendon healing with GDF 5 and 6. Acta Orthop Scand 70:51-54, 1999
- Baskin DS, Ryan PG, Westmark RM, et al: Anterior cervical discectomy fusion and plating with cornerstone-SR: rhBMP-2 vs autograft (abstract). AANS/CNS Joint Section on Spine and Peripheral Nerves, February 2002, Orlando, FL, p 26, 2002
- Boden SD: Biology of lumbar spine fusion and use of bone graft substitutes: Present, future, and next generation. Tissue Eng 6:383-399, 2000
- Boden SD, Martin GJ, Jr., Horton WC, et al: Laparoscopic anterior spinal arthrodesis with rhBMP-2 in a titanium interbody threaded cage. J Spinal Disord 11:95-101, 1998
- Boden SD, Martin GJ, Jr., Morone MA, et al: Posterolateral lumbar intertransverse process spine arthrodesis with recombinant human bone morphogenetic protein 2/hydroxyapatite-tricalcium phosphate after laminectomy in the nonhuman primate. Spine 24:1179-1185, 1999
- Boden SD, Schimandle JH, Hutton WC: An experimental lumbar intertransverse process spinal fusion model. Radiographic, histologic, and biomechanical healing characteristics. Spine 20:412-420, 1995
- Boden SD, Titus L, Hair G, et al: Lumbar spine fusion by local gene therapy with a cDNA encoding a novel osteoinductive protein (LMP-1). Spine 23:2486-2492, 1998
- Brodke DS, Gollogly S, Alexander Mohr R, et al: Dynamic cervical plates: Biomechanical evaluation of load sharing and stiffness. Spine 26:1324-1329, 2001
- Celeste AJ, Iannazzi JA, Taylor RC, et al: Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci USA 87:9843-9847, 1990
- Chen D, Harris MA, Rossini G, et al: Bone morphogenetic protein 2 (BMP-2) enhances BMP-3, BMP-4, and bone cell differentiation marker gene expression during the induction of mineralized bone matrix formation in cultures of fetal rat calvarial osteoblasts. Calcif Tissue Int 60:283-290, 1997
- Constam DB, Robertson EJ: Regulation of bone morphogenetic protein activity by pro domains and proprotein convertases. J Cell Biol 144:139-149, 1999
- Cook SD, Dalton JE, Tan EH, et al: In vivo evaluation of recombinant human osteogenic protein (rhOP-1) implants as a bone graft substitute for spinal fusions. Spine 19:1655-1663, 1994
- Derynck R: TGF-beta-receptor-mediated signaling. Trends Biochem Sci 19:548-553, 1994
- Derynck R, Zhang Y: Intracellular signaling: The mad way to do it. Curr Biol 6:1226-1229, 1996
- Dickman CA, Gornet MF, Burkus JK, et al: A prospective randomized lumbar fusion study comparing rhBMP-2 with autograft for cage ALIF (abstract). Congress of Neurological Surgeons, September 29-October 4, 2001, San Diego, CA, p 135, 2001
- Frazen A, Heldin NE: BMP-7-induced cell cycle arrest of anaplastic thyroid carcinoma cells via p21(CIP1) and p27(KIP1). Biochem Biophys Res Commun 285:773-781, 2001
- Ghosh-Choudhury N, Woodruff K, Qi W, et al: Bone morphogenetic protein-2 blocks MDA MB 231 human breast cancer cell proliferation by inhibiting cyclin-dependent kinase-mediated retinoblastoma protein phosphorylation. Biochem Biophys Res Commun 272:705-711, 2000
- Grauer JN, Patel TC, Erulkar JS, et al: 2000 Young Investigator Research Award. Evaluation of OP-1 as a graft substitute for intertransverse process lumbar fusion. Spine 26:127-133, 2001
- Griffith DL, Keck PC, Sampath TK, et al: Three-dimensional structure of recombinant human osteogenic protein 1: Structural paradigm for the transforming growth factor beta superfamily. Proc Natl Acad Sci USA 93:878-883, 1996
- Hecht BP, Fischgrund JS, Herkowitz HN, et al: The use of recombinant human bone morphogenetic protein 2 (rhBMP-2) to promote spinal fusion in a nonhuman primate anterior interbody fusion model. Spine 24:629-636, 1999
- Helm GA, Alden TD, Sheehan JP, et al: Bone morphogenetic proteins and bone morphogenetic protein gene therapy in neurological surgery: A review. Neurosurgery 46:1213-1222, 2000
- Hjertner O, Hjorth-Hansen H, Borset M, et al: Bone morphogenetic protein-4 inhibits proliferation and induces apoptosis of multiple myeloma cells. Blood 97:516-522, 2001
- Holliger EH, Trawick RH, Boden SD, et al: Morphology of the lumbar intertransverse process fusion mass in the rabbit model: A comparison between two bone graft materials—rhBMP-2 and autograft. J Spinal Disord 9:125-128, 1996
- Honda Y, Knutsen R, Strong DD, et al: Osteogenic protein-1 stimulates mRNA levels of BMP-6 and decreases mRNA levels of BMP-2 and -4 in human osteosarcoma cells. Calcif Tissue Int 60:297-301, 1997
- Israel DI, Nove J, Kerns KM, et al: Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors 13:291-300, 1996
- Lacroix P: Recent investigations on the growth of bone. Nature 156:576-583, 1945
- Lieberman JR, Daluiski A, Stevenson S, et al: The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am 81:905-917, 1999
- Lieberman JR, Le LQ, Wu L, et al: Regional gene therapy with a BMP-2-producing murine stromal cell line induces heterotopic and orthotopic bone formation in rodents. J Orthop Res 16:330-339, 1998
- Lovell TP, Dawson EG, Nilsson OS, et al: Augmentation of spinal fusion with bone morphogenetic protein in dogs. Clin Orthop 243:266-274, 1989
- Luyten FP, Cunningham NS, Ma S, et al: Purification and partial amino acid sequence of osteogenin, a protein initiating bone differentiation. J Biol Chem 264:13377-13380, 1989
- Martin GJ, Jr, Boden SD, Titus L: Recombinant human bone morphogenetic protein-2 overcomes the inhibitory effect of ketorolac, a nonsteroidal anti-inflammatory drug (NSAID), on posterolateral lumbar intertransverse process spine fusion. Spine 24:2188-2194, 1999
- Martin GJ, Jr, Boden SD, Titus L, et al: New formulations of demineralized bone matrix as a more effective graft alternative in experimental posterolateral lumbar spine arthrodesis. Spine 24:637-645, 1999
- Masuda K, Takegami K, An H, et al: Recombinant osteogenic protein-1 upregulates extracellular matrix metabolism by rabbit annulus fibrosis and nucleus pulposus cells. Trans Orthop Res Soc 24:1029-1035, 1999
- Mayer H, Scutt AM, Ankenbauer T: Subtle differences in the mitogenic effects of recombinant human bone morphogenetic proteins-2 to -7 on DNA synthesis on primary bone-forming cells and identification of BMP-2/4 receptor. Calcif Tissue Int 58:249-255, 1996
- Miyazono K, ten Dijke P, Heldin CH: TGF-beta signaling by Smad proteins. Adv Immunol 75:115-157, 2000
- Morone MA, Boden SD: Experimental posterolateral lumbar spinal fusion with a demineralized bone matrix gel. Spine 23:159-167, 1998
- Morone MA, Boden SD, Hair G, et al: The Marshall R. Urist Young Investigator Award. Gene expression during autograft lumbar spine fusion and the effect of bone morphogenetic protein 2. Clin Orthop 351:252-265, 1998
- Muschler GF, Hyodo A, Manning T, et al: Evaluation of human bone morphogenetic protein 2 in a canine spinal fusion model. Clin Orthop 308:229-240, 1994
- Orui H, Imaizumi S, Ogino T, et al: Effects of bone morphogenetic protein-2 on human tumor cell growth and differentiation: A preliminary report. J Orthop Sci 5:600-604, 2000
- Ozkaynak E, Rueger DC, Drier EA, et al: OP-1 cDNA encodes an osteogenic protein in the TGF-beta family. EMBO J 9:2085-2093, 1990
- Ozuna R, Sandhu HS, Kanim LEA, et al: Carriers for bone morphogenetic protein for posterolateral spinal fusion. North American Spine Society 10th Annual Meeting, October 1995, Washington DC, p 14, 1995
- Patel TC, Erulkar JS, Grauer JN, et al: Osteogenic protein-1 overcomes the inhibitory effect of nicotine on posterolateral lumbar fusion. Spine 26:1656-1661, 2001
- Sakou T: Bone morphogenetic proteins: From basic studies to clinical approaches. Bone 22:591-603, 1998
- Sampath TK, Reddi AH: Homology of bone-inductive proteins from human, monkey, bovine, and rat extracellular matrix. Proc Natl Acad Sci USA 80:6591-6595, 1983
- Sandhu HS, Kanim LE, Kabo JM, et al: Effective doses of recombinant human bone morphogenetic protein-2 in experimental spinal fusion. Spine 21:2115-2222, 1996
- Sandhu HS, Kanim LE, Kabo JM, et al: Evaluation of rhBMP-2 with an OPLA carrier in a canine posterolateral (transverse process) spinal fusion model. Spine 20:2669-2682, 1995
- Schimandle JH, Boden SD, Hutton WC: Experimental spinal fusion with recombinant human bone morphogenetic protein-2. Spine 20: 1326- 1337, 1995
- Sellers RS, Peluso D, Morris EA: The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. J Bone Joint Surg Am 79:1452-1463, 1997
- Sellers RS, Zhang R, Glasson SS, et al: Repair of articular cartilage defects one year after treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2). J Bone Joint Surg Am 82:151-160, 2000
- Senn N: On the healing of aseptic bone cavities by implantation of antiseptic decalcified bone. Am J Med Sci 98:219-240, 1889
- Shaffrey CI, Polly DW, Ogilvie JW, et al: Rh-BMP-2 a cost analysis from a hospital and payers perspective (abstract). AANS/CNS Joint Section on Spine and Peripheral Nerves, February 2002, Orlando, FL, p 37, 2002
- Suh DY, Boden SD, Louis-Ugbo J, et al: Delivery of recombinant human bone morphogenetic protein-2 using a compression-resistant matrix in posterolateral spine fusion in the rabbit and in the non-human primate. Spine 27:353-360, 2002
- Toriumi DM, Kotler HS, Luxenberg DP, et al: Mandibular reconstruction with a recombinant bone-inducing factor. Functional, histologic, and biomechanical evaluation. Arch Otolaryngol Head Neck Surg 117:1101-1112, 1991
- Urist MR: Bone: Formation by autoinduction. Science 150:893-899, 1965
- Wolfman NM, Hattersley G, Cox K, et al: Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-beta gene family. J Clin Invest 100:321-330, 1997
- Wozney JM: The bone morphogenetic protein family: Multifunctional cellular regulators in the embryo and adult. Eur J Oral Sci 106:160-166, 1998
- Wozney JM, Rosen V, Celeste AJ, et al: Novel regulators of bone formation: Molecular clones and activities. Science 242:1528-1534, 1988
- Yasko AW, Lane JM, Fellinger EJ, et al: The healing of segmental bone defects, induced by recombinant human bone morphogenetic protein (rhBMP-2). A radiographic, histological, and biomechanical study in rats. J Bone Joint Surg Am 74:659-670, 1992
- Yoon ST, Boden SD: Osteoinductive molecules in orthopaedics: Basic science and preclinical studies. Clin Orthop 395:33-43, 2002
- Zdeblick TA, Ghanayem AJ, Rapoff AJ, et al: Cervical interbody fusion cages. An animal model with and without bone morphogenetic protein. Spine 23:758-766, 1998
