A Screening Test for Cognitive and Affective Functioning in School-Age Children with Traumatic Brain Injury
George P. Prigatano, PhD
Saurabh Gupta, MC
Vicky T. Lomay, PhD
Division of Neurology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
Neuropsychological screening tests should sample cognitive and affective functions. A “downward extension” of the BNI Screen for Higher Cerebral Functions was developed for school-age children (i.e., BNI Screen for Higher Cerebral Functions-Children). The test was administered to 18 age-matched trauma controls, 22 children with mild TBI, and 20 children with moderately severe to severe TBI. As predicted, on the BNI Screen for Higher Cerebral Functions-Children, the Total score was reliably lower and the time needed to complete the test was reliably longer in children with a moderately severe to severe TBI compared to the trauma controls and children with a mild TBI. As predicted, the scores of the trauma controls and children with mild TBI on the BNIS-C did not differ in this postacute sample. The findings suggest that the BNI Screen for Higher Cerebral Functions-Children may be a clinically useful screening test of higher integrated brain functions for children with a TBI.
Key Words: affect, cognition, neuropsychology testing, traumatic brain injury
Abbreviations used: ANOVA, analysis of variance; CT, computed tomography, GCS, Glasgow Coma Scale; MR, magnetic resonance; TBI, traumatic brain injury; SD, standard deviation; WISC-III, Wechsler Intelligence Scale for Children-Third Edition
Few screening tests are available to assess neuropsychological functioning in school-age children.[2,18] Time limitations and a child’s clinical status may preclude a lengthy and comprehensive evaluation,[2] but screening tests for children are also potentially useful. During the screening phase of a neuropsychological examination, information about a child’s overall level of functioning and areas of specific strengths or weaknesses can help clinicians to plan tests that need to be administered and the sequence of testing. Furthermore, children with TBI are often evaluated soon after injury and may be followed months or years after injury.[8] A screening test that can be administered to such children repetitively could be useful from a clinical perspective, particularly for planning rehabilitation activities. We therefore introduced the BNI Screen for Higher Cerebral Functions for School-Age Children (BNIS-C), a “downward extension” of the BNI Screen for Higher Cerebral Functions,[23] which was developed for adults.
In the current study, we used the BNIS-C to investigate three hypotheses. First, children with moderately severe to severe TBI were predicted to obtain lower Total scores on the BNIS-C than age-matched children with mild TBI or trauma controls. Based on the neuropsychological literature on mild TBI,[1,13] postacute children with mild TBI (i.e., an average of 9 to 12 months after injury) were expected to perform at a level comparable to trauma controls. Therefore, postacute patients with mild TBI were predicted to have a Total BNIS-C score that did not significantly differ from those of trauma controls. Children with moderately severe to severe TBI typically perform slower on a variety of neuropsychological tests.[3,7,13] Therefore, in the third hypothesis, the total time to complete the BNIS-C was predicted to be longer in children with a moderately severe to severe TBI compared to the age-matched trauma controls and children with mild TBI.
Methods
Subjects
Sixty children involved in an ongoing study on parental perceptions on recovery after TBI at St. Joseph’s Hospital and Medical Center, Phoenix, Arizona, served as subjects. All children were in school at the time of the study and between the ages of 7 and 14 years. The inclusion criteria were as follows: English as the primary language, no prior history of TBI, and no pretrauma history of psychiatric and neurological disorders based on parental reports.
Three groups of children were studied. The first group consisted of 20 children with moderately severe to severe TBI. Severe TBI was defined as having an admitting GCS score between 3 and 8.[30] If no GCS score was available in the hospital records, severe TBI was based on duration of coma (i.e., unresponsiveness) for at least 24 hours as documented in the hospital records. Moderately severe TBI was defined as having an admitting GCS score of 9 to 12 with a clear space-occupying lesion in the brain as reflected on CT or MR imaging, or an associated skull fracture.
The second group consisted of 22 children who were classified as having mild TBI. Mild TBI was defined as an admitting GCS score between 13 and 15 with a positive loss of consciousness or, at minimum, “confusion” at the time of injury. None of these children had positive findings on CT or skull x-rays.
The third group consisted of 18 trauma controls. A trauma control was defined as a child who had suffered an orthopedic injury that resulted in evaluation at the Emergency Department. None of these children were diagnosed as having suffered a TBI. On admission, however, all children were assessed with a GCS score.
Procedures
All children were administered the BNIS-C[24] in addition to selected subtests of the WISC-III[31] by a board-certified clinical neuropsychologist, a resident in clinical neuropsychology, or a graduate psychology student trained to administer these tests.
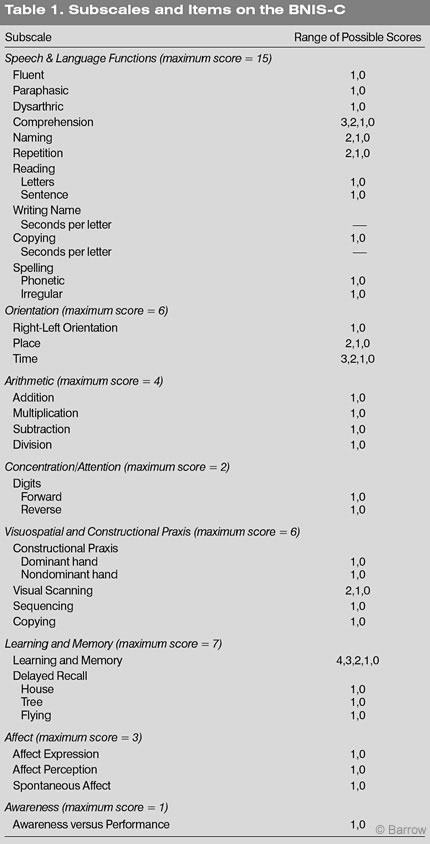
The rationale and initial validation studies of the BNIS-C are described elsewhere.[24] Test-retest reliability has also been documented.[26] The test consists of eight subtests (Speech and Language Functions, Orientation, Arithmetic, Concentration/Attention, Visuospatial and Constructional Praxis, Learning and Memory, Affect, and Awareness; Table 1). Both subtest scores and a Total score are calculated. The latter is the sum of subtest scores plus three screening items that reflect the child’s level of consciousness, level of cooperation, and level of basic language skills to understand the instructions of the test. The total possible score is 50 points. The time needed to complete the BNIS-C is also calculated.
The Vocabulary, Block Design, and Coding subtests from the WISC-III were also administered to obtain an estimate of the child’s basic knowledge of words, visuospatial abilities, and speed of new learning.
The children’s age at testing, time since injury (i.e., chronicity), handedness, gender, and admitting GCS scores were compared across groups. The level of education of the children’s mother was also obtained to estimate the comparability of the socioeconomic background of the three groups of children.[6]
Statistical Analysis
Data were analyzed using commercially available software (Statistical Package for Social Science, Inc., Chicago, IL). An ANOVA for independent groups was used to analyze the BNIS-C Total score and time to complete the task. A one-way ANOVA was calculated for each subtest score on the BNIS-C. No hypotheses were tested regarding potential group differences on the subtests.
Demographic variables were compared using one-way ANOVA. The Bonferroni correction was used to correct for multiple comparisons. Chisquare analyses were conducted to identify differences in the frequency of handedness and gender among the three groups.
Results
On average, children were 10.5 years old at the time of testing. There were no significant differences in age among the groups (F=.059, df =2, p=.943). However, chronicity (i.e., time since injury at testing) did differ among the groups (F=5.584, df =2, p=.006). When tested, children with a mild TBI and the trauma controls were slightly less than 1 year posttrauma. These children, however, are still considered to be in the “postacute stage.” The children with moderately severe to severe TBI were also in the postacute stage: On average 2.69 years had elapsed since their injury (Table 2).
Groups did not differ in terms of gender (?2=1.489, df =2, p=.457) or the frequency of handedness (?2 =6.723, df =4, p=.151). Most children were right handed. The educational level of the children’s mothers also was comparable across the three groups (F=1.585, df =2, p=.215). On admission the moderately severe to severe group had reliably lower GCS scores (F=102.4, df =2, p=.00) than trauma controls and children with mild TBI.
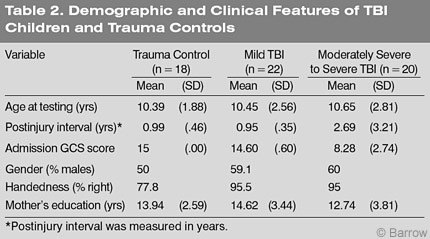
Performance on the Coding subtest of the WISC-III, which measures speed of information processing, was decidedly inferior in children with moderately severe to severe TBI (F=13.550, df =2, p=.00; Table 3). In fact, their lowest score was on the Coding subtest. Their performance was also significantly worse on the Vocabulary subtest (F= 3.335, df =2, p=.043) and on the Block Design subtest (F=4.766, df =2, p= .012). Collectively, these data indicate that children with mild TBI and the trauma controls performed in the average range on each of the three subtests of the WISC-III. These findings are consistent with the literature.[7,13]
The BNIS-C Total score of children with moderately severe to severe TBI was significantly worse than that of the trauma controls and children with mild TBI (F=6.307, df =2, p=.003; Table 4). However, the scores of the trauma controls and mild TBI patients did not significantly differ (Table 4). Their scores were within one point of each other and standard deviations were comparable. These findings support both Hypothesis 1 and 2.
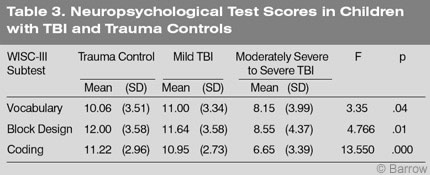
The total time to complete the BNIS-C was significantly longer in children with moderately severe to severe TBI compared to the trauma controls and children with mild TBI (F=6.30, df =2, p=.034). Thus, Hypothesis 3 was also supported.
There were a number of other group differences (Table 4). Children with moderately severe to severe TBI performed worse on the Speech and Language subtest, the Visuospatial/Constructional Praxis subtest, the Learning and Memory subtest, and the Affect subtest. None of these differences, however, were predicted.
Discussion
To obtain reliable information during the neuropsychological examination of a child, the clinician must establish rapport and engage the child in the testing procedures. Introductory tasks must engage the child’s interest and provide a mechanism for meaningful dialogue between the child and examiner. Baron[2] emphasized the importance of choosing tests that children can solve at the beginning of a neuropsychological examination. Screening tests should therefore provide children with ample opportunity to succeed before they are presented more challenging tasks.
Furthermore, developmental psychologists have long emphasized the intimate connection between children’s affective state and their willingness to take cognitive tests. The temperament of children and their willingness to separate from a caregiver provide important information about their personality[14] and about how readily they may be engaged in a neuropsychological examination.[7] Although this aspect of the BNIS-C was not evaluated, the test was constructed to be socially and culturally meaningful to children.
Cognitive Performance After TBI
Children with a TBI can exhibit a variety of cognitive disturbances. Disturbances in working memory;[16] speed of new learning and speed of motor performance; 3 decreased performance on standardized tests of intelligence, especially the WISC-III;[7,13] and various cognitive abilities considered executive functions[6] are common. Given the exploratory nature of this investigation, no hypotheses were made about how the performance of the children might have differed on the various subtests of the BNIS-C. Interestingly, however, the children with moderately severe to severe TBI obtained significantly lower scores on the Speech and Language subtest, the Visuospatial/Constructional Praxis subtest, and the Learning and Memory subtest. Thus, three “cognitive” subtests of the BNIS-C reliably differentiated children with moderately severe to severe TBI from children with mild TBI and trauma controls. These findings are compatible with the literature on neuropsychological impairments associated with pediatric TBI.
The poor performance of the children with severe TBI on the Visuospatial/ Constructional Praxis subtest is consistent with the literature on the neuropsychological disturbances associated with pediatric TBI. More than 40 years ago Diller and Birch[11] recognized that visuospatial disturbances were common after various types of brain injury during childhood. Jaffe et al.[13] noted that Performance IQ seemed especially vulnerable to severe TBI and was clearly below normal limits after injury. This finding has also been reported by other investigators.[7] Likewise, learning and memory difficulties are common after pediatric TBI.[13]
In the present study, the performance of children with mild TBI did not differ from that of the trauma controls on the Speech and Language subtest. However, the performance of children with severe TBI did. The presence of subtle but persistent speech and language disturbances in nonaphasic TBI children may be especially important. Levin et al.[17] and others[15] have reported word fluency disturbances in these children. Dennis and Barnes[9,10] have documented a variety of subtle but important disturbances in linguistic processing and output in children with mild to severe TBI.
Affective Performance After TBI
Existing screening tests have focused on cognitive functioning and do not sample a child’s ability to express and perceive affect.[28] However, children with a TBI can exhibit a variety of disturbances in affect. Such children have a high incidence of pre- and postinjury psychiatric disturbances.[20,22,29] Changes in personality are especially noted when a measurable decline in intelligence is documented.[21] Psychosocial adjustment is clearly affected by severity of initial brain injury.[12]
On a subtest of the BNIS-C intended to sample affect (i.e., the ability to generate affect in one’s tone of voice and to perceive facial affect), children with moderately severe to severe TBI performed significantly worse than the children with mild TBI and trauma controls. The Affect subtest also samples the spontaneity of emotional expression in response to a humorous figure. These findings have been reported in adults with TBI but have not been studied in children with TBI. In the present study, however, the findings of the school-age children with moderately to severe TBI along this dimension were similar to those of adults with TBI.[4,5]
During the screening process, sampling both cognitive and affective functioning of school-age children is important because the findings have clear implications for rehabilitation. In adults, for example, improvements in both cognitive and affective functioning are predictive of rehabilitation outcome.[27] Further investigation of these relationships in children, both in the acute and postacute stages of injury, seems warranted.
Developmental Perspective
Recent standardization data on 232 children between the ages of 6 and 14 years provide evidence that the BNISC is developmentally sensitive (i.e., older children obtain higher scores).[25] Assessing neuropsychological functions from a developmental perspective may be useful. Diller and Birch[11] emphasized this important principle, which is seldom recognized in contemporary neuropsychological assessment.
Despite the limitations of downward-extension tests,[2] they also offer a developmental perspective that may be especially important for neuropsychological diagnosis and rehabilitation planning.[11,19] The major task of childhood is to prepare children for life as adults. Thus, tests that sample abilities that develop throughout the lifespan could provide important information not only for predicting neurorehabilitation outcome but for predicting long-term performances in numerous areas. This information is especially relevant to the field of brain injury rehabilitation for children and adults.
Conclusions
Initial studies on the reliability and validity of the BNIS-C have been promising.[24,26] The findings from the present study provide initial support that the BNIS-C may also be useful for sampling cognitive and affective functions in postacute school-age children with a history of moderately severe to severe TBI. As predicted, these children obtained a lower BNIS-C Total score than the trauma controls or children with mild TBI. Also as predicted, the performance of the children with mild TBI and the trauma controls did not differ significantly. Finally, children with moderately severe to severe TBI were decidedly slower in completing the test than the other two groups of children. Collectively, these findings add to both the construct and concurrent validity of this screening test.
Acknowledgments
Funding for this research project was obtained through the Arizona Department of Health Services, Office for Children with Special Healthcare Needs, Contract HP261032-001 through the sponsorship of the LBB Legacy Foundation. The authors also thank Camea Gagliardi, MEd; Susan Borgaro, PhD; Pam Goslar, PhD; and Gifford Loda for their support in conducting this study. Special thanks to Eve DeShazer and Mary Henry for their assistance in preparing this manuscript.
References
- Asarnow RF, Satz P, Light KZ, et al: The UCLA study of mild closed head injury in children and adolescents, in Broman SH, Michel ME (eds): Traumatic Head Injury in Children. New York: Oxford University Press, 1995, pp 117-146
- Baron IS: Neuropsychological Evaluation of the Child. New York: Oxford University, 2004
- Bawden HN, Knights RM, Winogron HW: Speeded performance following head injury in children. J Clin Exp Neuropsychol 7:39-54, 1985
- Borgaro SR, Prigatano GP: Early cognitive and affective sequelae of traumatic brain injury: A study using the BNI Screen for Higher Cerebral Functions. J Head Trauma Rehabil 17:526-534, 2002
- Borgaro SR, Prigatano GP, Kwasnica C, et al: Cognitive and affective sequelae in complicated and uncomplicated mild traumatic brain injury. Brain Inj 17:189-198, 2003
- Brookshire B, Levin HS, Song J, et al: Components of executive function in typically developing and head-injured children. Dev Neuropsychol 25:61-83, 2004
- Chadwick O, Rutter M, Shaffer D, et al: A prospective study of children with head injuries: IV. Specific cognitive deficits. J Clin Neuropsychol 3:101-120, 1981
- Complair PA, Butler RW, Lezak MD: Providing psychological services to families of brain-injured adults and children in the present health-care environment, in Prigatano GP, Pliskin NH (eds): Clinical Neuropsychology and Cost Outcome Research: A Beginning. New York: Psychology Press, 2003, pp 83-107
- Dennis M, Barnes MA: Speech acts after mild or severe childhood head injury. Aphasiology 14:391-405, 2000
- Dennis M, Barnes MA: Comparison of literal, inferential, and intentional text comprehension in children with mild or severe closed head injury. J Head Trauma Rehabil 16:456-468, 2001
- Diller L, Birch HG: Psychological evaluation of children with cerebral damage, in Birch HG (ed): Brain Damage in Children: The Biological and Social Aspects. New York: Williams and Wilkins, 1964, pp 27-43
- Fletcher JM, Ewing-Cobbs L, Miner ME, et al: Behavioral changes after closed head injury in children. J Consult Clin Psychol 58:93-98, 1990
- Jaffe KM, Polissar NL, Fay GC, et al: Recovery trends over three years following pediatric traumatic brain injury. Arch Phys Med Rehabil 76:17-26, 1995
- Kagan J, Snidman N: The Long Shadow of Temperament. Cambridge, MA: The Belknap Press of Harvard University, 2004
- Kinsella G, Prior M, Sawyer M, et al: Neuropsychological deficit and academic performance in children and adolescents following traumatic brain injury. J Pediatr Psychol 20:753-767, 1995
- Levin HS, Hanten G, Zhang L, et al: Changes in working memory after traumatic brain injury in children. Neuropsychology 18:240-247, 2004
- Levin HS, Song J, Ewing-Cobbs L, et al: Word fluency in relation to severity of closed head injury, associated frontal brain lesions, and age at injury in children. Neuropsychologia 39:122-131, 2001
- Lezak MD, Howieson DB, Loring DW, et al: Neuropsychological Assessment. New York: Oxford University Press, 2004
- Luria AR: The Making of Mind. A Personal Account of Soviet Psychology. Cambridge,MA: Harvard University, 1979
- Max JE: Psychiatric Sequelae of Traumatic Brain Injury in Children and Adolescents. AAPM & R Conference Proceedings: Phoenix, 2004
- Max JE, Koele SL, Castillo CC, et al: Personality change disorder in children and adolescents following traumatic brain injury. J Int Neuropsychol Soc 6:279-289, 2000
- Max JE, Robin DA, Lindgren SD, et al: Traumatic brain injury in children and adolescents: Psychiatric disorders at two years. J Am Acad Child Adolesc Psychiatry 36:1278-1285, 1997
- Prigatano GP: BNI Screen for Higher Cerebral Functions: Rationale and initial validation. BNI Quarterly 7:2-9, 1991
- Prigatano GP: The BNI Screen for Children: Rationale and initial validation studies. Barrow Quarterly 20:27-32, 2004
- Prigatano GP, Gagliardi CJ: BNI Screen for Higher Cerebral Functions in School–Age Children: A Manual for Administration and Scoring. Phoenix: Barrow Neurological Institute, 2005
- Prigatano GP, Gupta S, Loman VT: Test-retest reliability of the BNI Screen for Higher Cerebral Function for School-Age Children. Barrow Quarterly 21:22-24, 2005
- Prigatano GP, Wong JL: Cognitive and affective improvement in brain dysfunctional patients who achieve inpatient rehabilitation goals. Arch Phys Med Rehabil 80:77-84, 1999
- Reitan RM: Aphasia and Sensory-Perceptual Deficits in Children. Tucson: Neuropsychology Press, 1984
- Shaffer D, Chadwick O, Rutter M: Psychiatric Outcome of Localized Head Injury in Children. Amsterdam: Excerpta Medica, 1975
- Teasdale G, Jennett B: Assessment of coma and impaired consciousness. A practical scale. Lancet 2:81-84, 1974
- Wechsler D: WISC-III. Wechsler Intelligence Scale for Children-Third Edition. Manual. San Antonio: The Psychological Corporation, Harcourt Brace Jovanovich, 1991