
Intracerebral Schwannomas: Case Report and Review of the Literature
Eric M. Horn, MD, PhD
Joseph M. Zabramski, MD
Guiseppe Lanzino, MD†
Stephen W. Coons, MD‡
Divisions of Neurological Surgery and ‡Neuropathology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
†Current Address: University of Illinois, Peoria, Illinois
Abstract: Intracerebral schwannomas are rare tumors of the CNS. We present a 21-yearold woman with a right parieto-occipital schwannoma. The etiology of these tumors is unknown, but because most reported cases have involved young patients, a developmental origin has been suggested. It has also been hypothesized that these tumors originate from the Schwann cells associated with trigeminal nerve fibers that innervate the dura. These theories and literature related to this case are reviewed.
Key Words: etiology, intracerebral, neurofibromatosis, schwannomas, supratentorial
Abbreviations Used: CNS, central nervous system; CT, computed tomography; MR, magnetic resonance
Intracerebral schwannomas are rare CNS tumors. Including the present case, only 65 cases have been reported in patients without neurofibromatosis.[2,4,6,7,9,14,16] The cellular origin of these tumors is unknown. Speculation varies from a congenital abnormality during development of the CNS to seeding from trigeminal fibers innervating the dura.[3,5] We report a 21-year-old woman with a supratentorial intracerebral schwannoma and review the literature with reference to these theories and differences in location and age of presentation.
Case Report
A 21-year-old woman with a 5-week history of bilateral headache sought treatment in the emergency department. She also had experienced a few days of nausea, vomiting, and blurry vision. She described having a kidney disorder as a child that had resolved and a history of reactive airway disease. She had no family history of neurological disorders or cancer.
She had no abnormalities on general physical examination. Her neurological examination was remarkable only for incomplete left homonymous hemianopsia. She had no stigmata of neurofibromatosis.
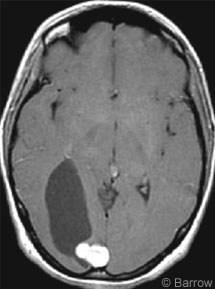
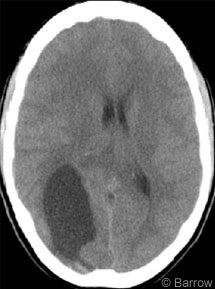
The patient underwent CT, which showed a 3 x 5-cm hypodensity in the right parieto-occipital cortex (Fig. 1). On MR imaging a cystic mass with a 2 x 1.5-cm nodule adjacent to the falx strongly enhanced after a gadolinium injection (Fig. 2). On the basis of the patient’s history and diagnostic imaging studies, surgery was recommended.
Treatment
The tumor was approached via a bioccipital craniotomy using MR imaging stereotactic image guidance (StealthStation®, Medtronic SNT, Louisville, CO). Before the dura was opened, a ventriculostomy catheter was placed stereotactically to drain the cystic portion of the mass and to relax the brain. The tumor, which appeared to arise from the midline falx, was resected with the exception of a small portion that involved the lateral wall of the sagittal sinus. That portion was extensively coagulated with bipolar cauterization.
Pathological Findings
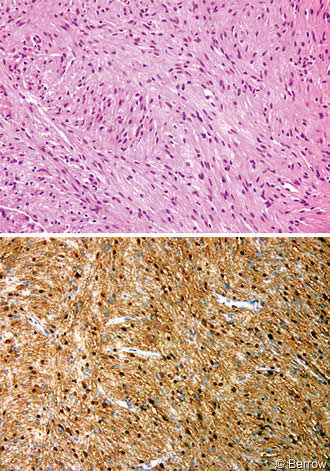
Frozen-section analysis indicated a low-grade spindle-cell neoplasm that favored the diagnosis of a schwannoma or meningioma. Tumor specimens were fixed in formalin and processed routinely. Light microscopy showed a moderately cellular spindle cell neoplasm that was organized in intersecting fascicles (Fig. 3A). The eosinophilic fibrillary stroma was variably compact (Antoni A) or loosely textured (Antoni B). Atypia was generally mild and no mitotic figures were identified. The tumor cells were diffusely immunoreactive for S-100 protein (Fig. 3B) and demonstrated multifocal patchy positivity for glial fibrillary acidic protein, a pattern common in intracranial schwannomas. The tumor cells were negative for epithelial membrane antigen, effectively excluding meningioma from the diagnosis. A reticular stain demonstrated a rich fibrillary network that highlighted the fascicular architecture. This finding was inconsistent with pleomorphic xanthoastrocytoma and pilocytic astrocytoma. The microscopic features were characteristic of schwannoma.[8,11,15] The special stains were performed because the location of the lesion was unusual and because of the need to exclude other tumors that might have this appearance on gross examination and imaging studies.
Outcome
Postoperatively, the patient did well and had no new deficits. On postoperative Day 3, she was discharged to home. At her 3-month follow-up examination, she was doing well and reported significant visual improvement. At that time MR imaging showed no evidence of residual or recurrent tumor.
Discussion
Intracerebral schwannomas are rare lesions. Only 65 cases have been reported in the literature.[2,4,6,7,9,14,16] In addition to their location, the other main feature that differentiates these lesions from schwannomas arising from the vestibular and trigeminal nerves is the patient’s age at presentation. Acoustic and trigeminal schwannomas typically manifest in the fifth or sixth decade,[13] while intracerebral schwannomas manifest in the third or fourth decade.
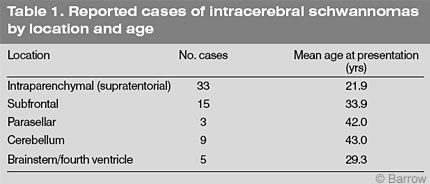
This difference in age is even more dramatic when subfrontal and infratentorial schwannomas are excluded and only intraparenchymal supratentorial schwannomas are considered (Table 1). The latter have been reported 33 times in patients with a mean age of 21.9 years (median, 18 years). This finding has led to speculation that the origin of intracerebral schwannomas differs from that of acoustic schwannomas. However, the age of patients with intraparenchymal subfrontal, parasellar, and infratentorial schwannomas at diagnosis (32 cases; mean age, 36.8 years; median, 38 years) is similar to that associated with acoustic schwannomas. These lesions most likely originate in a similar fashion as acoustic schwannomas but in different locations.
The origin of intraparenchymal schwannomas has been debated since their discovery.[5] One hypothesis is that these tumors represent a developmental abnormality. The strongest evidence for this argument is the young age of patients at presentation. Scant molecular or histological evidence, however, supports this hypothesis. Histologically, the mesenchymally derived pial cells can resemble Schwann cells, suggesting that an abnormality during development may cause these cells to grow slowly into a schwannoma.[10] It is also possible that the aberrant migration of neural crest cells during development could lead to a collection of Schwann cells in the brain parenchyma.[3] The abnormal development of Schwann cells in the subventricular zone, which is the major source of multipotent cell types in the CNS, could lead to tumor originating from these cells.[1]
Intraparenchymal schwannomas, distinct from the subfrontal, sellar, and infratentorial locations, most likely are present from early development. They arise in younger patients than the other intracerebral schwannomas and are usually deep in the parenchyma, where the abnormally developed Schwann cells are thought to originate. This mechanism differs from that underlying acoustic schwannomas, which develop de novo from an abnormality on chromosome 22 in Schwann cells located on the vestibular nerve.[12] To further support this hypothesis, however, genetic studies are needed to determine if a different molecular abnormality is associated with intraparenchymal schwannomas.
Another hypothesis concerning the origin of intracerebral schwannomas is that they may arise de novo from an aberrant conversion of a Schwann cell present in or near the brain parenchyma. The innervation of the meninges arises mainly from branches of the trigeminal nerves, which have a myelin sheath composed of Schwann cells. The age at diagnosis of tumors most closely associated with the dura (i.e., subfrontal and sellar schwannomas) is similar to that of acoustic neuromas, suggesting that the origin of subfrontal and sellar schwannomas is related to the Schwann cells of the dural or anterior ethmoidal nerves.
Conclusion
The current case of an intracerebral schwannoma in a young patient supports the hypothesis that these lesions originate as a result of a developmental abnormality. The origin of these tumors may vary based on the patient’s age at presentation and location of the lesion.
References
- Akiyama Y, Honmou O, Kato T, et al: Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol 167:27-39, 2001
- Erongun U, Ozkal E, Acar O, et al: Intracerebral schwannoma: Case report and review. Neurosurg Rev 19:269-274, 1996
- Frim DM, Ogilvy CS, Vonsattal JP, et al: Is intracerebral schwannoma a developmental tumor of children and young adults? Case report and review. Pediatr Neurosurg 18:190-194, 1992
- Gelabert M, Fernandez J, Lopez E: [Schwannoma of the olfactory groove]. Neurologia 15:404-405, 2000
- Gibson AA, Hendrick EB, Conen PE: Case reports. Intracerebral schwannoma. Report of a case. J Neurosurg 24:552-557, 1966
- Huang PP, Zagzag D, Benjamin V: Intracranial schwannoma presenting as a subfrontal tumor: Case report. Neurosurgery 40:194-197, 1997
- Husain M, Mishra UK, Newton G, et al: Isolated olfactory groove neurilemmoma. Surg Neurol 37:115-117, 1992
- Louw D, Sutherland G, Halliday W, et al: Meningiomas mimicking cerebral schwannoma. J Neurosurg 73:715-719, 1990
- Praharaj SS, Vajramani GV, Santosh V, et al: Solitary olfactory groove schwannoma: Case report with review of the literature. Clin Neurol Neurosurg 101:26-28, 1999
- Russel DS, Rubenstein LJ: Pathology of Tumours of the Nervous System. London: Edward Arnold, 1989
- Schnitt SJ, Vogel H: Meningiomas. Diagnostic value of immunoperoxidase staining for epithelial membrane antigen. Am J Surg Pathol 10:640-649, 1986
- Seizinger BR, Martuza RL, Gusella JF: Loss of genes on chromosome 22 in tumorigenesis of human acoustic neuroma. Nature 322:644-647, 1986
- Selesnick SH, Jackler RK, Pitts LW: The changing clinical presentation of acoustic tumors in the MRI era. Laryngoscope 103:431-436, 1993
- Tan TC, Ho LC, Chiu HM, et al: Subfrontal schwannoma masquerading as meningioma. Singapore Med J 42:275-277, 2001
- Winek RR, Scheithauer BW, Wick MR: Meningioma, meningeal hemangiopericytoma (angioblastic meningioma), peripheral hemangiopericytoma, and acoustic schwannoma. A comparative immunohistochemical study. Am J Surg Pathol 13:251-261, 1989
- Zagardo MT, Castellani RJ, Rees JH, et al: Radiologic and pathologic findings of intracerebral schwannoma. AJNR Am J Neuroradiol 19:1290-1293, 1998
