
Neuroimaging Innovation Center
Mission and Focus
The mission of the Barrow Neuroimaging Innovation Center focuses on the following three areas:
- Advancing imaging technology for improving patient diagnosis and care
- Serving as an imaging resource for the Barrow and St. Joseph’s community and the greater research community
- Providing education in medical imaging
Our center encompasses 5,000 square feet of space dedicated to imaging research. The center was built to bring together clinical, academic, and industry expertise to produce robust clinical imaging technology and bring it to market. To that end, it houses faculty and researchers of the Division of Neuroimaging Research, Arizona State University graduate students, a scientist from Philips Healthcare, and works with collaborators across the Phoenix Valley as a multi-institutional team in pursuit of this common goal. We are also a member of the North American Imaging in MS Cooperative (NAIMS).
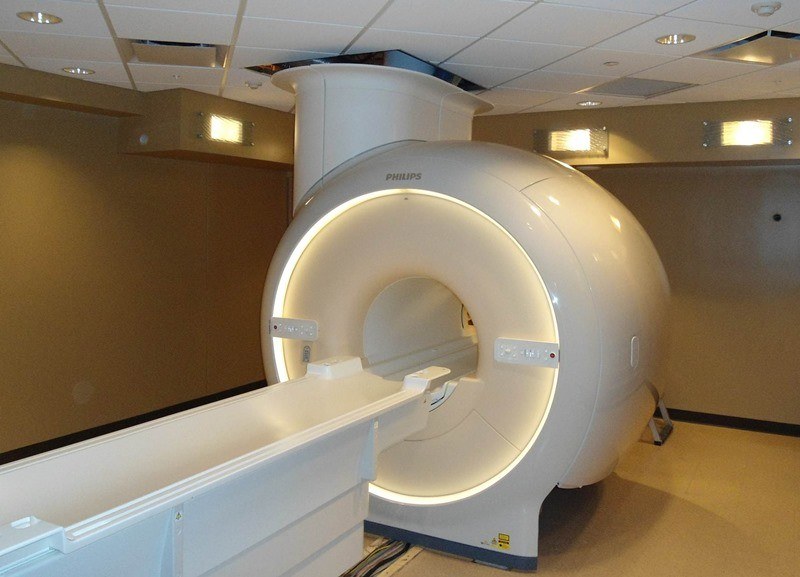
We operate a whole body 3-Tesla Philips Ingenia MRI scanner dedicated entirely to research. Our staff works with researchers from Barrow or any other institution wishing to utilize this scanner for their biomedical research. For information on scheduling, please fill use the email address listed below.

Contact Information
Barrow Neuroimaging Innovation Center
350 West Thomas Road
Phoenix, Arizona 85013
The 3T MRI is equipped with a host of imaging capabilities to meet a variety of scanning needs, including:
- Anatomical Imaging. A variety of scans can be used to highlight almost any anatomy of interest. The latest technology provides high-resolution, accurate assessment of morphometry and volume.
- Functional MRI (fMRI). A variety of presentation hardware, including goggles and earphones, can be used to present stimulus to a subject in order to assess brain activation.
- Quantitative Flow and Diffusion Imaging. Advanced MR methods can measure and quantify motion as subtle as water diffusion as well as flow velocity in 3 dimensions, either in vivo or in physical models using a programmable MR compatible pump.
- Diffusion Tensor Imaging (DTI). In vivo measurement of the anisotropic diffusion of water is used to generate images of white matter tracts in the brain.
- Perfusion Imaging. The measurement of the effects of perfusion and contrast agents is a valuable tool for examining neuropathologies such as brain tumors and stroke. Endogenous contrast methods (e.g., arterial spin labeling, ASL) and contrast agent bolus tracking methods (dynamic susceptibility contrast and dynamic contrast enhanced MRI) are some of the methods available to researchers using the Center.
- Angiography. The visualization of vascular anatomy is important for the study of vascular disease, stenosis, and blood-flow occlusion. High-resolution in vivo imaging of vessels is possible with the use of specialized imaging techniques, contrast agents, or both.
- Susceptibility Weighted Imaging (SWI). SWI is a method that is sensitive to variations in a tissue’s local magnetic fields and can be used to enhance visualization of deoxyhemoglobin in veins, iron deposition in the brain, hemorrhages, microbleeds and calcification. SWI is currently used for a range of clinical applications including stroke, TBI and a range of neurodegenerative disorders.
All Contacts
Richard Dortch, PhD
Associate Professor
Division of Neuroimaging Research
Director, Barrow Neuroimaging Innovation Center
Richard.Dortch@BarrowNeuro.org
Sharmeen Maze RT(R)(MR)
Research MR Technologist
Sharmeen.Maze@DignityHealth.org
(602) 406-2644
Lori Steffes
Department Research Coordinator
Lori.Steffes@DignityHealth.org
(602) 406-4215
Barrow Neuroimaging Innovation Center
350 West Thomas Road
Phoenix, Arizona 85013
