Differential Response Characteristics of Patients with Nonepileptic and Epileptic Seizures on a Test of Verbal Learning and Memory
Authors
Jennifer J. Bortz, PhD
George P. Prigatano, PhD
David Blum, MD
Robert S. Fisher, MD, PhD
Division of Neurology, Barrow Neurological Institute, Mercy Healthcare Arizona, Phoenix, Arizona
Abstract
Expert clinicians and empirical investigators have found it difficult to distinguish patients with nonepileptic seizures from those with true epileptic seizures using quantitative measures of neuropsychological test performance. We examined qualitative response characteristics on the California Verbal Learning Test of 41 patients undergoing continuous video-audio electroencephalographic monitoring in an effort to distinguish these patient groups (12 patients with left temporal foci, 11 with right temporal foci, and 18 with nonepileptic seizures). Patients with nonepileptic seizures explicitly recognized fewer target words compared to those with epileptic seizures. In addition, patients with nonepileptic seizures rarely made false-positive errors, which resulted in failure to endorse a significant number of items on the recognition list. This response tendency is called a negative response bias. In contrast, patients with left temporal foci endorsed a high number of items on the recognition test, which produced a positive response bias. Patients with right temporal foci demonstrated no consistent response tendency. A negative response bias index (i.e., cutoff score <0) showed a specificity of 61% and a sensitivity of 91%. Failure to explicitly recognize words after repeated exposures may reflect aspects of psychological denial in patients with nonepileptic seizures. Response bias indices may thus help identify patients with nonepileptic seizures and help to explain the psychological mechanisms underlying this complex disorder.
Key Words : epilepsy, neuropsychology, nonepileptic seizures, pseudoseizure
Many patients referred to epilepsy centers for management of intractable seizures do not have epilepsy but do have one of the imitators of epilepsy, most commonly pseudoseizures.24 Pseudoseizures, also called psychogenic seizures or nonepileptic seizures, can be difficult even for expert clinicians to distinguish from epileptic seizures. Since the causes, treatment, and prognosis of these two conditions differ substantially,18,20,30 their distinction is important.
The diagnosis of nonepileptic seizures is complicated by several factors, including (1) overlap in the phenomenology of epileptic seizures and nonepileptic seizures, (2) a high incidence of head injury with a history of seizures in nonepileptic seizure patients, (3) electroencephalographic (EEG) abnormalities in both patient populations, and (4) a high comorbidity rate of seizures and nonepileptic seizures in the same patient.17,37 In addition, the psychiatric features of patients with nonepileptic seizures are diverse and include a high incidence of depression (also prevalent in patients with epileptic seizures), conversion hysteria, somatoform disorders, anxiety, various characterological disorders, and malingering.8,21,30
Overlap in neurologic signs and symptoms and the heterogeneity of psychiatric features of nonepileptic seizures have limited the utility of neuropsychological tests in the differential diagnosis of nonepileptic seizures. Patients with nonepileptic seizures and those with epileptic seizures do not consistently differ on such measures as the revised Wechsler Adult Intelligence Scale (WAIS-R) Verbal and Performance IQ scores or on overall measures of impairment obtained from the Halstead-Reitan Neuropsychological Test Battery.11,37 However, investigators in previous studies assessed quantitative differences in overall level of performance. Qualitative aspects of the performance of patients with nonepileptic seizures have not been examined systematically.
In considering the usefulness of clinical history and brief measures of psychopathology in characterizing nonepileptic seizures, Trimble34 emphasized the potential “value of seeking information beyond the phenomenology of the seizure in making a diagnosis.” Little information is available on response characteristics of patients with nonepileptic seizures. However, attention to qualitative features of neuropsychological test performance has contributed to the differential diagnosis of other clinical groups presenting with similar levels of memory impairment. Kaszniak,15 for example, proposed that qualitative features of memory performance often separate patients with late-life depression from those with true dementia. Depressed elderly individuals frequently demonstrate a “don’t know” response tendency and may evidence considerable difficulty on more “effortful” tasks.25, 35 This pattern reflects aconservative response bias, characterized by fewer false-positive errors and fewer true-positive responses compared to both healthy control subjects and neurologically impaired patients. In contrast, patients with dementia tend to produce a lenient response bias characterized by a high number of false-positive and intrusion errors. Intrusion errors also are common in other patient populations with known brain dysfunction.4
The present investigation examined qualitative differences in the response characteristics of patients with epileptic seizures and those with nonepileptic seizures on a verbal learning and memory task. We hypothesized that these two groups of patients would not differ in the actual number of words recalled on the free and cued-recall portions of the California Verbal Learning Test (CVLT)5 but would differ in their response style and associated frequency of intrusion errors. Like depressed patients, patients with nonepileptic seizures were predicted to demonstrate a conservative, or negative, response bias on the CVLT recognition subtest. In contrast, patients with epileptic seizures were predicted to show a lenient response strategy, evidenced by a positive response bias on the recognition task and a greater number of intrusion errors across learning and recall trials relative to patients with nonepileptic seizures.
Finally, since verbal memory deficits are more common after left hemisphere damage, patients with left temporal seizure foci were predicted to demonstrate a stronger positive response bias and to make more intrusion errors relative to patients with seizure onset localized to right temporal structures.
Methods
Subjects
Forty-one patients were referred for neuropsychological evaluation while undergoing continuous video-audio EEG monitoring for the observation, classification, and treatment of medically intractable seizures. Thirty-two channel scalp EEG recordings were saved via magnetic tape and time-locked to video and auditory super-VHS color recordings. Computerized detection algorithms “flagged” suspicious segments of the EEG for the staff to review. All clinical events were reviewed in detail by the attending neurologists, and any EEG alterations were correlated with behavioral changes.
Of the 41 patients, 23 were determined to have focal temporal lobe seizure onsets and were subsequently considered appropriate candidates for surgical treatment of intractable epilepsy. Twelve patients demonstrated a prominence of abnormal electrophysiological activity originating in left temporal (LT) structures, and 11 had predominantly right temporal (RT) foci. Of the 41 patients, 18 were diagnosed with nonepileptic seizures. No significant EEG correlates of “typical” behavioral spells were documented during the monitoring of these 18 patients. This finding, concordant with cumulative diagnostic information evaluated by attending neurologists, led to the diagnosis of nonepileptic seizures.
There were no significant differences between patients with nonepileptic seizures and those with epileptic seizures in age, sex, education, or Full Scale IQ (Table 1).
Procedures
The CVLT was administered to all patients as part of a comprehensive neuropsychological evaluation while they were on the Epilepsy Monitoring Unit. The CVLT attempts to measure both the process of verbal learning and the amount of material learned.5 Multiple indices quantify recall and learning characteristics, recall errors, and recognition memory abilities.
Administration of the CVLT begins with the examiner’s description of the task and subsequent presentation of a randomly ordered list of 16 words that can be classified into four unique semantic categories: spices and herbs, fruits, tools, and clothing. This list, described as Monday’s shopping list, is presented in five learning trials. The subjects’ free-recall responses are recorded after each presentation. Upon completing the fifth trial, subjects are presented with a different list of 16 words, described as Tuesday’s shopping list. These words fall under the superordinate categories of fruits, spices, kitchen tools, and types of fish. Subjects’ spontaneous recall of the interference list is recorded, immediately followed by recording the subjects’ free-recall of the initial word list. Cued recall is then assessed by instructing subjects to recall target words from the original list according to semantic categories provided by the examiner (e.g., Tell me the shopping items that were spices and herbs . . . types of tools, etc.). After a 20-minute delay, subjects’ free and cued recall of the first word list is again documented. Finally, recall is tested in a recognition format. The examiner reads a list of 44 words, consisting of the 16 target items and 4 types of distractors: items appearing on the second word list, items from the same semantic category, phonemically similar items, and items ostensibly unrelated to the target words. Subjects indicate whether a given word appeared on the original list by responding Yes or No immediately after the examiner presents each word.
Dependent Measures
Three CVLT indices were used to assess qualitative features of the subjects’ task performance: response bias, false-positive errors, and total intrusion errors. The response bias index reflects a Yes or No response tendency on the CVLT recognition subtest, independent of the type of recognition item. This index was therefore used as the primary indicator of a lenient vs. conservative response strategy. The response bias index is calculated as the number of false-positive errors minus the number of misses, divided by the sum of the number of false positive errors and misses. False-positive errors were words endorsed by the patient on the recognition subtest that were not target items from the original word list. Because target words can be grouped according to distinct semantic categories, false-positive errors on the CVLT typically reflect within-category errors or emerge as same-category words from the distractor list.
Patient groups also were compared according to the total number of intrusion errors. Intrusion errors on the CVLT consist of words reported by the patient during free and cued-recall tasks that were not included in the target list. Similar to the response bias index, this measure reflects problems in differentiating relevant from irrelevant responses (CVLT manual, p. 31), with a high number of intrusion errors indicating problems in response discrimination. Thus, a lenient response strategy could be reflected by a high number of false-positive responses on the recognition subtest, or by a high number of intrusion errors elicited during free and cued-recall trials.
Results
Primary Findings
Two response characteristics differentiated nonepileptic seizure, LT, and RT patient groups. Consistent with the hypothesis that patient groups would differ in response strategies, only patients with nonepileptic seizures evidenced a negative response bias (Table 2). Specifically, the mean response bias scores of patients with nonepileptic seizures were significantly lower than LT patients, and the scores of RT patients fell between these two groups. Also as hypothesized, patients with epileptic seizures made more false-positive errors compared to patients with nonepileptic seizures on the recognition subtest of the CVLT, with a significantly greater number of false-positive errors made by LT compared to patients with nonepileptic seizures [F (2, 38) = 7.34, p = .0020].
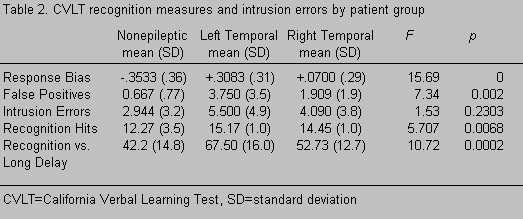
Patient groups did not differ with respect to mean number of intrusion errors [F (2, 38) = 1.53, p = .2303], although the pattern was consistent with predicted differences between groups (i.e., x¯ = 2.94, 4.09, and 5.50, for nonepileptic seizure, RT, and LT groups, respectively). Statistical outliers were present in the distribution of the intrusion scores in the nonepileptic seizure and RT groups. Consequently, intrusion scores more than two standard deviations from their group mean were excluded from repeat analysis.† Scheffé comparisons revealed that LT patients made significantly more intrusion errors than patients with nonepileptic seizures [x¯ = 5.5, 3.3, and 2.0, for LT, RT, and nonepileptic seizure groups, respectively; F(2, 35) = 3.83, p = 0.0313].
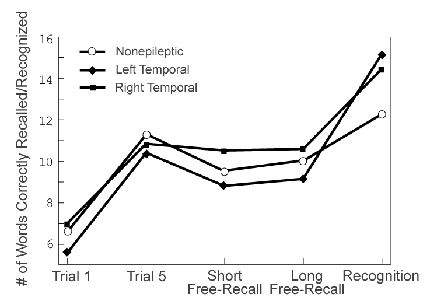
A priori predictions regarding contrast measures of the CVLT were not made. However, patients with nonepileptic seizures performed significantly worsethan LT patients on the long delay vs. recognition hits index (x¯ = 42.2 and 67.5, respectively; Table 2). This index compares the number of words spontaneously recalled after a 20-minute delay (i.e.,long-delay free recall) to the number of target words correctly identified on the recognition subtest. Figure 1 shows the mean number of words recalled on key recall tasks by patients with nonepileptic seizures and those with epileptic seizures. Given the well-documented finding of superior recognition vs. free-recall performance in both neurologically impaired and normal control populations,6 the opposite pattern demonstrated by patients with nonepileptic seizures may be important. Finally, no differences were found between patients with nonepileptic seizures and those with epileptic seizures on standard immediate or delayed recall measures (Table 3).
Additional Comparisons
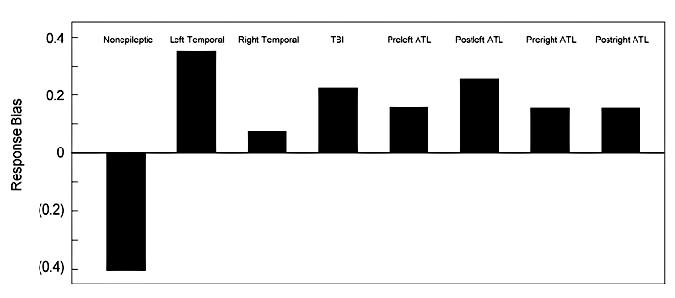
To further explore the significance of the response bias finding, we also compared the groups’ data with data from patients with traumatic brain injuries (TBI) seen at our institute and with data reported by Hermann and colleagues†† (Figs. 2 and 3).12 Only patients with nonepileptic seizures demonstrated a negative response bias. These data support the potential usefulness of response characteristics and other recognition memory indices in differentiating epileptic from nonepileptic seizures. In the LT group, seven patients showed a positive response bias (58.3%) and five showed a neutral response bias (41.7%). None of the LT patients produced a negative response bias score. Four RT patients showed a positive response bias (36.4%), five showed a neutral response bias (45.4%), and two showed a small negative response tendency (18.2%). In contrast, only one patient with nonepileptic seizures showed a positive response bias (5.6%) and six patients exhibited a neutral response bias (33.3%). Of the 18 patients with nonepileptic seizures, 11 (61%) showed a negative response bias.
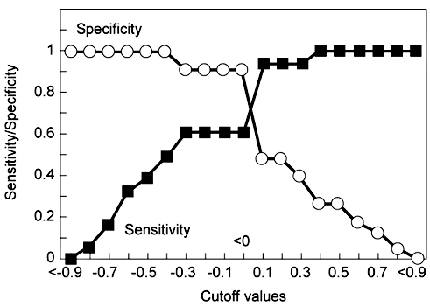
Sensitivity and Specificity
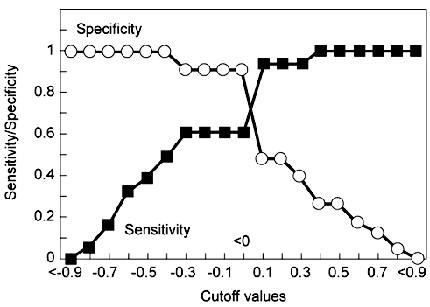
The sensitivity of a diagnostic test indicates how well the measure places patients into the correct diagnostic category—in this case, correctly classifying patients with nonepileptic seizures as having nonepileptic seizures. The specificity of a test indicates how well the measure rejects patients who should not be placed into the diagnostic category—in this case, rejecting patients with epileptic seizures from the classification of nonepileptic seizures. Using a cut-off score of 0 (i.e., a negative response bias), the overall sensitivity of the response bias index was 61% with a specificity of 91% (Fig. 4).
Discussion
In the present study, qualitative response characteristics distinguished nonepileptic seizures from epileptic seizures whereas standard measures of learning and memory did not.
Negative vs. Positive Response Biases
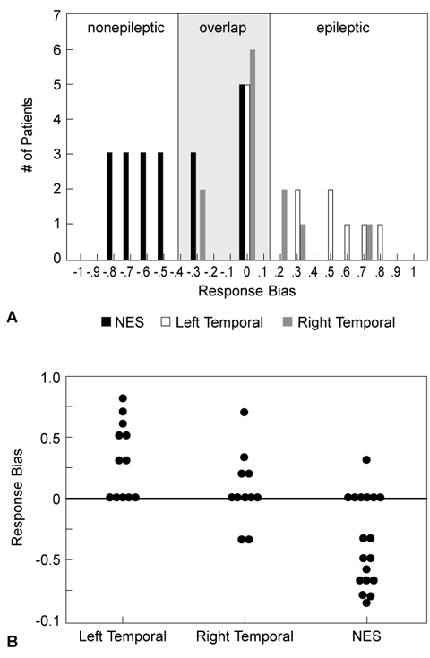
A positive response bias is obtained when patients consistently report that words presented during the recognition trial belong to the target list (Monday’s list on the CVLT) when, in fact, most did not. In contrast, a negative response bias is obtained when patients indicate that few of the recognition items belonged to the original target list when, in fact, 16 of these words were presented across 5 consecutive learning trials.
Hermann et al.12 observed a slight positive response bias in patients with intractable epilepsy evaluated before and after anterior temporal lobectomy (Fig. 2). We documented positive response biases in patients with epileptic seizure as well as in the group of TBI patients. Such errors may result from inefficient encoding strategies, from problems with interfering stimuli influencing patients’ responses, as well as from a susceptibility to endorse semantically and/or phonemically similar test items. In certain clinical groups, a positive response bias may therefore be one signature of true memory impairment.
In contrast, a negative response bias is less common in patients with a mild or moderate memory impairment attributable to a neurological disease. Only patients with nonepileptic seizures failed to recognize a relatively high percentage of the 16 target words, despite repeated exposure to these words. Severely amnestic patients might be expected to show a negative response bias (e.g., patients unable to recall administration of the memory task). In epileptic patients who have partial memory disturbances but not a frank amnestic syndrome, the presence of a negative response bias may alert the clinician that factors other than memory impairment could be responsible for the patient’s poor test performance.
Other neurologic groups, particularly patients with frontal lobe impairment, also may show a negative response bias. Frontal lobe injuries produce various forms of sequencing impairments, including deficits in temporal ordering,22, 23 that could produce a negative response bias because of interference or an inability to discriminate whether items were on the target or interference list (i.e., Monday’s vs. Tuesday’s shopping list). This distinction is important because patients with frontal lobe partial seizures are at particularly high risk of being diagnosed with nonepileptic seizures.3, 31 Scores on the CVLT discriminability index (which reflects signal vs. noise discrimination on the recognition task), measures of proactive interference, and semantic clustering indices of the CVLT may help identify patients with dysfunction of the frontal systems.
Depression and Nonepileptic Seizures
Patients with major affective disorders exhibit conservative response strategies. Memory complaints are common among depressed individuals, who as noted, often perform poorly on formal tests of mnestic functions.13, 14, 38 Performance deficits may partially result from motivational disturbances because depressives perform fairly well on “automatic” tasks but poorly on tasks that require sustained effort.7 For example, Weingartner and colleagues35 reported a high inverse correlation between impairment on “effortful” memory tasks and scores on the Hamilton Depression Rating Scale and on the Beck Depression Inventory. Our study does not address the specificity of a negative response bias in primary psychiatric populations, although the comorbidity of depression and nonepileptic seizures is high. Thus, the negative response bias tendency demonstrated by our patients with nonepileptic seizures might be explained by motivational deficits associated with depression or by related problems with sustained attention.
At least two reasons, however, suggest that concomitant depression cannot fully explain the negative response bias shown by patients with nonepileptic seizures. First, the comorbidity of depression and epileptic seizures is also high, particularly in patients with LT foci.2, 32 Some LT patients, therefore, would be expected to show a negative response bias, but none of our LT patients demonstrated this response tendency. Second, the response bias index is derived solely from responses elicited during the recognition subtest of the CVLT. Recognition memory tasks are inherently easier than free recall and thus require less effort than other forms of memory tests. Recognition requires only a decision about whether a given stimulus was present at encoding, whereas recall requires independent lexical access, retrieval of response alternatives, and the recognition decision.28
Why, then, did patients with nonepileptic seizures perform worse than patients with epileptic seizures on the recognition (hits) vs. long-delay contrast measure? As noted, this index compares the number of words spontaneously recalled on the 20-minute delayed free-recall task to the number of target words endorsed on the subsequent recognition trial. As anticipated, both LT and RT patients performed better on the recognition trial than on the free-recall trial (Fig. 1). Patients with nonepileptic seizures did not. Specifically, LT patients recalled an average of six more words on the recognition trial (38%) than did patients with nonepileptic seizures. RT patients showed a similar, though less dramatic, improvement by correctly identifying four additional words on the recognition task (25%). In contrast, patients with nonepileptic seizures recalled on average only two additional words on the recognition task (12%). This pattern seems difficult to attribute solely to “motivational” or “effort- related” deficits and suggests that other factors are involved. We propose an alternative hypothesis based primarily upon clinical observation to explain these findings. Assuming that nonepileptic seizures result from psychological, rather than from organic dysfunction (e.g., a conversion disorder), the tendency for patients with nonepileptic seizures to not verbally report or explicitly recognize information repeatedly presented to them may reflect the psychological defense mechanism of “denial.”16, 29
Denial and Nonepileptic Seizures
In denial, a psychological experience is not recognized when the reality of the event or circumstance may be fairly obvious to others. Denial as a psychological defense is distinguished from what has been termed organically based unawareness of deficit after brain injury.19, 27 In the latter case, the patient is believed to lack conscious representation of an event because key neurological centers are damaged.
We do not suggest that psychological denial is an overinclusive explanation for nonepileptic seizures, although the literature indicates that this mechanism is prominent in a significant number of patients. Nonepileptic seizures represent the second most frequent symptom profile of conversion disorders.33 Moreover, several case reports9, 10, 36 and a recent prospective investigation1 have documented a relatively high incidence of sexual trauma in patients with nonepileptic seizures. Such a history would certainly be compatible with psychological denial or repression.
A history of sexual trauma was, in fact, elicited from 8 of 13 of our female patients with nonepileptic seizures. Although none of the five males reported histories of sexual abuse, psychiatric and clinical interviews revealed the following histories: childhood physical abuse (n=1), histories of an adolescent conduct disorder (n=2), and recurrent episodes of depression with reported suicide attempts that began in adolescence (n=2). Whether trauma histories were underreported by the males with nonepileptic seizures is unknown. All, however, had histories of psychological maladjustment that dated from childhood or adolescence. Also, many patients with nonepileptic seizures had not reported histories of abuse before their monitoring stay. It has been our experience that with the establishment of what might broadly be called the therapeutic alliance26 and attention to certain cues in the patient’s history (e.g., academic difficulties inconsistent with estimated intellectual abilities, and/or periods of frequent absences from school without obvious explanation), a more complete history may reveal important clinical and psychosocial information.
Conclusions
A negative response bias appears useful in distinguishing patients with nonepileptic seizures from those with true epileptic seizures. We do not, however, argue that this measure is independently or uniquely diagnostic of nonepileptic seizures. Patients’ response characteristics simply raise a diagnostic “flag” that factors other than a true neurologically based memory impairment may account for performance deficits on such measures as the CVLT. This finding offers insight about the cognitive and psychological strategies employed by patients with nonepileptic seizures. The actual sensitivity and specificity of this finding in the diagnosis of nonepileptic seizures will require further study of populations with other neurological and psychological disorders and of patients before and after treatment for their nonepileptic seizures.
Adapted from Bortz JJ, Prigatano GP, Blum D, Fisher RS: Differential response characteristics in nonepileptic and epileptic seizure patients on a test of verbal learning and memory. Neurology 45:2029-2034, 1995. Reprinted with permission of Little, Brown, and Company.
†Two patients from the group with nonepileptic seizures and one RT patient were excluded as outliers from the analysis.
††Data reproduced with permission from senior author.
References
- Alper K, Devinsky O, Perrine K, et al: Nonepileptic seizures and childhood sexual and physical abuse. Neurology 43:1950-1953, 1993
- Altshuler LL, Devinsky O, Post RM, et al: Depression, anxiety, and temporal lobe epilepsy. Laterality of focus and symptoms. Arch Neurol 47:284-288, 1990
- Boon PA, Williamson PD: The diagnosis of pseudoseizures [Review]. Clin Neurol Neurosurg 95:1-8, 1993
- Crosson B, Sartor KJ, Jenny AB, et al: Increased intrusions during verbal recall in traumatic and nontraumatic lesions of the temporal lobe. Neuropsychology 7:193-208, 1993
- Dellis DC, Kramer JH, Kaplan E, et al: California Verbal Learning Test. New York: Psychological Corporation: 1987
- Ellis H, Hunt RR: Fundamentals of Human Memory and Cognition. Dubuque, IA: Wm. C. Brown: 1983
- Ellis JC, Thomas RL, Rodriguez IA: Emotional mood states and memory: Elaborative encoding, semantic processing, and cognitive effort. J Exp Psychol Learn Mem Cogn 10:470-482, 1984
- Gates R, Erdahl P: Classification of non-epileptic events, in Rowan AJ, Gates JR (eds): Non-Epileptic Seizures. Stoneham, MA: Butterworth-Heinemann, 1993, pp 21-30
- Goodwin J, Simms M, Bergman R: Hysterical seizures: A sequel to incest. Am J Orthopsychiatry 49:698-703, 1979
- Gross M: Hysterical seizures: A sequel of incest, in Gross M (ed): Pseudoepilepsy: The Clinical Aspects of False Seizures. Lexington, MA: Lexington Books, 1983
- Hermann BP: Neuropsychological assessment in the diagnosis of non-epileptic seizures, in Rowan AJ, Gates JR (eds): Non-Epileptic Seizures. Stoneham, MA: Butterworth-Heinemann, 1993, pp 221-232
- Hermann BP, Wyler AR, Bush AJ, et al: Differential effects of left and right anterior temporal lobectomy on verbal learning and memory performance. Epilepsia 33:289-297, 1992
- Jackson RL, Smith LR: The effects of uncontrollable failure and depression on memorial processes. J Res Pers 118:463-479, 1984
- Johnson MH, Magaro PA: Effects of mood and severity on memory processes in depression and mania. Psychol Bull 101:28-40, 1987
- Kaszniak AW: Neuropsychological consultation to geriatricians: Issues in the assessment of memory complaints. Clin Neuropsychol 1:35-46, 1987
- Kubie LS: Practical and Theoretical Aspects of Psychoanalysis. New York: International Universities: 1950
- Lelliot PT, Fenwick P: Cerebral pathology in pseudoseizures. Acta Neurol Scand 83:129-132, 1991
- Lempert T, Schmidt D: Natural history and outcome of psychogenic seizures: A clinical study in 50 patients. J Neurol 237:35-38, 1990
- McGlynn SM, Schacter DL: Unawareness of deficits in neuropsychological syndromes [Review]. J Clin Exp Neuropsychol 11:143-205, 1989
- Meierkord H, Will B, Fish D, et al: The clinical features and prognosis of pseudoseizures diagnosed using video-EEG telemetry.Neurology 41:1643-1646, 1991
- Mendez MF, Cummings JL, Benson DF: Depression in epilepsy. Significance and phenomenology. Arch Neurol 43:766-770, 1986
- Milner B: Some effects of frontal lobectomy in man, in Warren JM, Akert L (eds): The Frontal Granular Cortex and Behavior. New York: McGraw-Hill, 1964, pp 313-334
- Milner B: Interhemispheric differences in the localization of psychological processes in man [Review]. Br Med Bull 27:272-277, 1971
- Porter RJ: Epileptic and non-epileptic seizures, in Rowan AJ, Gates JR (eds): Non-Epileptic Seizures. Stoneham, MA: Butterworth-Heinemann, 1993, pp 9-20
- Post F: Dementia, depression, and pseudodementia, in Benson DF, Blumer D (eds): Psychiatric Aspects of Neurologic Disease. New York: Grune & Stratton, 1975
- Prigatano GP, Klonoff PS, O’Brien KP, et al: Productivity after neuropsychologically oriented, milieu rehabilitation. J Head Trauma Rehabil 9:91-102, 1994
- Prigatano GP, Schacter L: Awareness of Deficit After Brain Injury: Theoretical and Clinical Issues. New York: Oxford University: 1991
- Rabinowitz JC, Mandler G, Patterson KE: Determinants of recognition and recall: Accessibility and generation. J Exp Psychol Gen 106:302-329, 1977
- Rapaport D, Gill MM, Schafer R: Diagnostic Psychological Testing. New York: International Universities: 1968
- Roy A: Pseudoseizures: A psychiatric perspective. J Neuropsychiatry Clin Neurosci 1:69-71, 1989
- Saygi S, Katz A, Marks DA, et al: Frontal lobe partial seizures and psychogenic seizures: Comparison of clinical and ictal characteristics. Neurology 42:1274-1277, 1992
- Taylor DC: Affective disorders in epilepsies: A neuropsychiatric review. Behav Neurol 2:49-68, 1989
- Toone BK: Disorders of Hysterical Conversion, in Bass C (ed): Somatization: Physical Symptoms and Psychological Illness. London: Blackwell, 1990
- Trimble MR: Pseudoseizures [Review]. Neurol Clin 4:531-548, 1986
- Weingartner H, Cohen RM, Murphy DL, et al: Cognitive processes in depression. Arch Gen Psychiatry 38:42-47, 1981
- Wilkus RJ, Dodrill CB: Factors affecting the outcome of MMPI and neuropsychological assessments of psychogenic and epileptic seizure patients. Epilepsia 30:339-347, 1989
- Wilkus RJ, Dodrill CB, Thompson PM: Intensive EEG monitoring and psychological studies of patients with pseudoepileptic seizures. Epilepsia 25:100-107, 1984
- Wolfe J, Granholm E, Butters N, et al: Verbal memory deficits associated with major affective disorders: A comparison of unipolar and bipolar patients. J Affect Disord 13:83-92, 1987