
High-Flow Dural Arteriovenous Malformations in Infants
Authors
Neil M. Borden, MD
Bruce L. Dean, MD
Richard A. Flom, MD
Burton P. Drayer, MD
Robert F. Spetzler, MD†
Division of Neuroradiology, †Division of Neurological Surgery, Barrow Neurological Institute, Mercy Healthcare Arizona, Phoenix, Arizona
Abstract
Dural arteriovenous malformations (AVMs) in infants are rare lesions that are difficult to eradicate and often require both endovascular and surgical management. These lesions are often characterized by high flow and high volume resulting in venous hypertension. Review of the literature and analysis of the following two case reports suggest that many of the clinical and imaging findings in infantile dural AVMs are a sequelae of venous hypertension. There can be many similarities with infants who harbor vein of Galen malformations, which may relate to the underlying intracranial venous hypertension.
Key Words : infantile dural AVM, vein of Galen malformation, venous hypertension
Dural arteriovenous malformations (AVMs) are thought to represent about 10 to 15% of all intracranial vascular malformations with arteriovenous shunting.1,25 Although most dural AVMs occur in adults, there are scattered reports of these lesions in neonates, infants, and children.1,4,9,15,26 Current opinion is that many dural AVMs in adults are related to underlying dural sinus thrombosis and recanalization13,22 although other factors have been implicated.17 Their occurrence in neonates and infants suggests a congenital origin in this subgroup of patients.1,9,15,17,25 In contrast to adults, pediatric patients with dural AVMs demonstrate more systemic and cranial signs and symptoms reflecting higher flow and higher volume shunts.1,11,15,17,25 These high-flow, high-volume shunts lead to venous hypertension. This process is similar to intracranial venous hypertension in infants with vein of Galen vascular malformations.18,21 We believe that venous hypertension in both groups of patients can cause similar clinical and imaging findings. We present two cases of high-flow infantile dural AVMs that demonstrate clinical signs and symptoms and findings on diagnostic imaging that we believe resulted from intracranial venous hypertension.
Illustrative Cases
Case 1
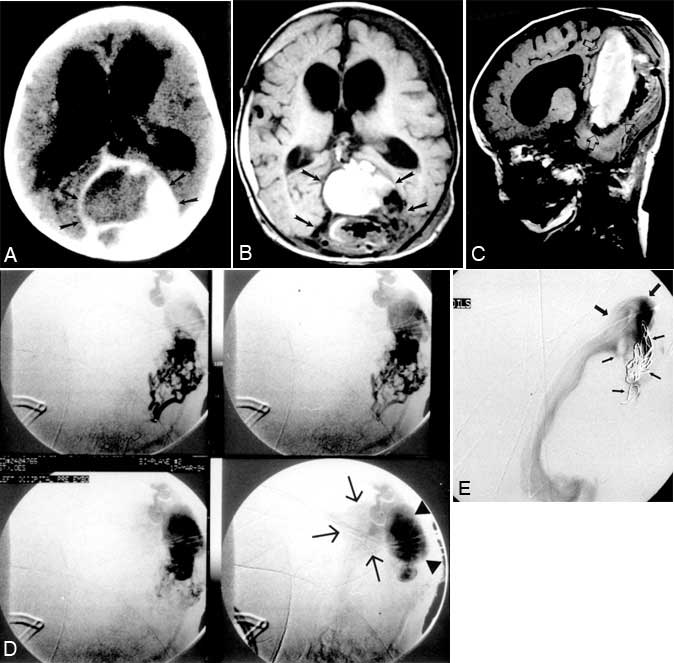
After an uneventful gestation and birth, a 4-month-old female presented to our institution with seizure and vomiting, left periorbital swelling since birth, borderline macrocephaly, and decreased motor development with poor head control. Conventional cerebral angiography at an outside institution demonstrated a large AVM in the region of the vein of Galen. Ventriculomegaly also was identified and led to placement of a right ventriculoperitoneal shunt. On examination, left periorbital soft-tissue swelling and proptosis, prominent scalp and forehead veins, and generalized muscle hypotonia were noted. Computed tomography (CT) demonstrated a large mass with vascular elements between the leaves of the dura of the posterior falx (Fig. 1A). There was prominent mass effect on the cerebellum and fourth ventricle, and calcifications were evident along the posterior aspect of the blood-filled mass. The left cavernous sinus and the left superior ophthalmic vein were enlarged asymmetrically.
Magnetic resonance (MR) imaging and MR venography revealed a large dural AVM with venous ectasia in the region of the torcula (Fig. 1B, C). The venous ectasia was partially thrombosed. This dilated recipient venous pouch drained into the left sigmoid sinus and a diminutive left internal jugular vein. Anomalous intra-axial deep venous structures appeared to drain anteriorly toward the left cavernous sinus and left orbit. Ventriculomegaly was present despite the previous shunt placement. Cerebral angiography demonstrated a large posterior fossa dural AVM fed by both middle meningeal arteries, both occipital arteries, the left accessory meningeal artery, and the posterior meningeal branch of the left vertebral artery. The venous drainage of this dural AVM was into a pouch-like structure projecting posteriorly and inferiorly from a massively dilated torcula. The drainage from the recipient venous pouch was into the torcula (Fig. 1D).
A multidisciplinary team involving neurosurgery, neuroradiology, and pediatric neurology was assembled to determine the optimal treatment for this infant. The team agreed to attempt preoperative embolization first to devascularize the high-flow shunt and to reduce the venous hypertension. Operative intervention to eradicate the lesion would follow.
The patient underwent transarterial particulate embolization of all of the feeding pedicles with good devascularization of the nidus. Polyvinyl alcohol (PVA) particles were used in all pedicles, and Gelfoam® powder was used in one pedicle since surgery would soon follow.
Four days after the initial transarterial embolization, the patient underwent an occipital craniotomy with partial skeletonization of the left transverse sinus. The malformation was too extensive and only partial surgical treatment was possible. Two days later, the infant underwent cerebral venography and partial embolization of the recipient venous pouch with placement of 12 platinum coils. Flow through the venous pouch was reduced slightly after transvenous coil embolization (Fig. 1E). Embolization decreased the orbital and venous swelling, but the infant’s neurological condition was unchanged.
Follow-up endovascular treatment with additional attempts to occlude the recipient venous pouch is planned. Further operative intervention also may be attempted.
Case 2
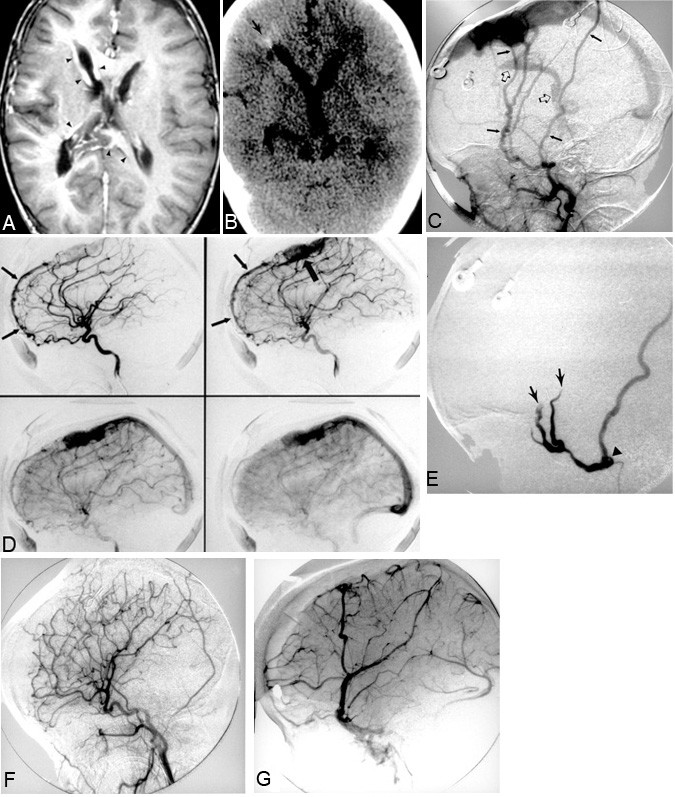
A 2-year-old male presented to the emergency room after he developed spontaneous seizure activity. The patient had a normal gestation to term and was delivered vaginally. No abnormalities were evident at or soon after birth. Several weeks before the child was admitted, however, the parents noticed congestion and puffiness of his right eyelid. At presentation, the child’s head circumference was 2.5 standard deviations above the mean for his age. Veins were prominent throughout the scalp, and the right periorbital region was swollen and congested. Chemosis of the right conjunctiva was present as well as questionable proptosis. He had no focal neurologic deficits. Abnormal dilatation of branches of the external carotid artery and the superior sagittal sinus on MR imaging, MR angiography, and MR venography suggested a dural AVM. The transcortical and subependymal veins and other sinuses also were engorged (Fig. 2A). The signal intensity of the white matter was abnormal. CT showed a large midline vascular structure in the frontal region consistent with the dilated superior sagittal sinus. White-matter calcifications in the periventricular zone of the right frontal region were present (Fig. 2B). Cerebral angiography confirmed a dural AVM near the superior sagittal sinus that was supplied by both middle meningeal arteries (Fig. 2C). The anterior falx branches of both ophthalmic arteries also contributed to the fistula (Fig. 2D). There was minimal supply from the distal right occipital artery.
Therapeutic planning was undertaken by a multidisciplinary team from neurosurgery, neuroradiology, and pediatric neurology. The team agreed upon preoperative embolization to facilitate surgical resection.
The patient underwent transarterial embolization of both the right and left middle meningeal arteries, which were found to feed the dural AV fistula located in the superior sagittal sinus region. PVA and straight platinum microcoils were used as embolic agents (Fig. 2E). After embolization, the patient underwent staged surgical resection. Complete resection was obtained after the fourth operation (Fig. 2F, G).
Operative intervention involved bilateral frontal craniotomy with skeletonization of the superior sagittal sinus dural arteriovenous fistula. Two re-explorations were necessary to eliminate the arterial feeders anteriorly. A third and final re-exploration on May 15, 1994 with posterior extension of the craniotomy flap was necessary to eliminate a posterior arterial feeder.
Postoperative angiography revealed complete obliteration of the dural AV fistula. No additional angiographic follow-up has been obtained to date. At a follow-up examination on April 17, 1995, the patient was free of seizures and off medications. His developmental progress was good and he had no focal neurologic deficits. Residual soft tissue swelling in the right periorbital region was present. Future angiographic evaluation is planned.
Discussion
The natural history of dural AVMs can be quite variable, depending on the location of the malformation, but most importantly, on the pattern of venous drainage.5 Although the symptomatology may be benign (i.e., tinnitus, bruit), patients can also become symptomatic with episodes of intracranial hemorrhage, seizures, focal neurologic deficits, and visual loss. According to Awad et al.,2 recruitment of leptomeningeal venous drainage (as in Case 2), variceal enlargement of recruited leptomeningeal veins, and Galenic venous drainage are associated with more aggressive dural AVMs. Classifying dural AVMs according to their pattern of venous drainage allows the natural history as well as the necessity for treatment of a specific lesion to be predicted. Cognard et al.5 revised and elaborated on Djindjian’s 1978 classification scheme. The revision provides a useful and practical approach in categorizing dural AVMs and provides data to help determine appropriate therapy in individual cases.
Dural AVMs in neonates and infants have only been reported sporadically. In contrast to typical dural AVMs in adults, most infantile lesions have high-flow rates and volumes.11,17 Consequently, these lesions often lead to intracranial venous hypertension and its sequelae. In infants and neonates, these sequelae can include headache, papilledema, seizures, focal neurologic deficits, developmental delay, proptosis, and eventually parenchymal injury with encephalomalacia. Elevated pressures in the dural venous sinuses are believed by many to contribute to hydrocephalus (communicating type) and macrocephaly, which is common in these cases.1,6,10,14,16-18,21,24,25
In a recent review, Cataltepe et all4 found only 13 reported cases of infantile dural AVMs. Infantile dural AVMs draining into or near the region of the torcula4,9,23,25,26 share angiographic similarities to the dural AVM in our Case 1. This region of the cranium is often involved in infantile dural AVMs.4,9,23,25,26 Clinical signs and symptoms of infantile dural AVMs described previously1,23,25,26 are strikingly similar to our cases and include macrocephaly, hydrocephalus, distended scalp veins, bruits, heart murmurs or heart failure, and delayed development. We believe the principal cause of many of these manifestations is underlying cerebral venous hypertension related to the high-flow arteriovenous shunting.
Elevated cerebral venous pressures may be the major physiological abnormality responsible for many of the clinical and imaging manifestations in children with vein of Galen vascular malformations.8,18,21 Infants with vein of Galen malformations often demonstrate ventriculomegaly. According to Mickle and Quisling,21 the etiology of the ventriculomegaly can be explained by expanded cerebral blood volume combined with elevated dural sinus pressures. The CSF resorptive capability is impaired, resulting in a communicating type of hydrocephalus.8,18,21 Direct mechanical compression of the aqueduct of Sylvius by the dilated vein may be a secondary and potentially aggravating mechanism for hydrocephalus in patients with vein of Galen malformations.18,21
The similarities in clinical and imaging findings between high-flow infantile dural AVMs and vein of Galen malformations are not surprising if, as we believe, underlying intracranial venous hypertension is present in both types of lesions. This similarity is illustrated in our two case reports.
Specific treatment regimens require a detailed knowledge of the angioarchitecture of the dural AVM: the specific location, the arterial feeders and, more importantly, the pattern of venous drainage. Patient symptomatology and potential morbidity of specific therapies also must be considered. The approach can be conservative in patients with tolerable symptoms (i.e., tinnitus, bruit) and with angiographically benign lesions (no leptomeningeal drainage). This strategy can involve either no treatment or low-risk arterial embolization. Arterial embolization often results in incomplete treatment of dural AVMs. It may reduce flow temporarily and ameliorate symptoms, but with time the signs and symptoms of the original lesion often reappear as collateral feeders are recruited. Recruitment of feeders after partial embolization can involve branches of the internal carotid artery or vertebrobasilar system, which are more difficult and potentially dangerous to treat. In contrast, patients with more aggressive clinical symptoms or with leptomeningeal venous drainage regardless of symptomatology should undergo complete eradication if possible.
Surgical intervention and/or endovascular techniques (transarterial and/or transvenous approach) have been utilized to treat dural AVMs.3,12,20 The use of stereotactic radiation in conjunction with embolization also has been described.19,20 Endovascular techniques sometimes provide a complete cure.11 The transvenous occlusion of the recipient venous pouch or outlet, when possible, appears to be more effective in treating certain dural AVMs.11
Transvenous endovascular obliteration of the venous structure collecting the flow of the dural AVM may be performed if the structure is a discrete, separate pouch or involves a segment of the dural sinus that does not function as a venous outlet for the normal brain. If, however, the structure also functions as a venous efferent for normal brain, it should not be closed because venous infarction could result.7,8 When a complete cure cannot be achieved by endovascular techniques, preoperative embolization can facilitate surgical resection by decreasing the vascularity of the lesion and hence reducing blood loss,1,3,12 which in turn may allow more complete resection and potentially shorten operative time.
Eradication of an infantile dural AVM is usually difficult. Embolization has rarely been used to treat infantile dural AVMs.1,26 Most of the treated infantile dural AVMs in these reports involved ligating the external carotid artery or common carotid artery.1,4,9,23 However, these cases were reported when ligation of feeding arteries was considered appropriate treatment for dural AVMs. Experience has taught us that this form of treatment is insufficient because collateral recruitment quickly re-establishes flow into the nidus of a dural AVM.3,20 Current treatment of dural AVMs often requires both embolization and surgical techniques as described in this report. In Case 2, complete obliteration of the dural AVM required four surgical stages (skeletonization) and one embolization. Case 1 required extensive transarterial and transvenous embolization and a surgical procedure (skeletonization) of the dural AVM. Because of its complexity, the dural AVM in Case 1 could not be cured surgically after embolization and so will be followed clinically.
Staged transvenous and transarterial embolization has emerged as the treatment of choice in vein of Galen vascular malformations.21 Embolization with obliteration of the dilated vein of Galen or median prosencephalic vein receiving the fistulous connections requires that the normal brain does not utilize this venous outlet. This principle of treatment applies to any vascular malformation when a transvenous endovascular approach is being contemplated.7,8 As mentioned, this principle also is applied to the treatment of dural AVMs.
The clinical presentation of dural AVMs in the pediatric population reflects the systemic and cranial effects of the high-flow state that elevates intracranial venous pressure.1,11,15,17,25 This elevated pressure may lead to communicating hydrocephalus and macrocephaly,1,6,10,14,16-18,21,24,25 venous engorgement with development of collateral pathways, venous ischemia with atrophic changes, white matter calcifications, focal neurologic deficits, and seizures. Cardiac symptoms in these cases also have been attributed to the high flow through the low-resistance shunt with secondary expansion of the circulating blood volume. These symptoms can manifest as heart murmurs or heart failure. Cranial bruits are often present in high-flow pediatric dural AVMs. In neonates, the clinical triad of congestive heart failure, macrocephaly, and a cranial bruit suggests an underlying vein of Galen vascular malformation.8,18,21,25 This clinical triad also has been described numerous times in neonates and infants with high-flow pial and dural AVMs1,6,9,25 and should be included in the differential diagnosis of these findings. Theoretically, any high-flow, low-resistance shunt, including parenchymal AVMs, could produce this clinical triad.
Intracranial venous hypertension can lead to a multitude of clinical and imaging findings. The two cases presented in conjunction with review of the literature strongly support our belief that venous hypertension is the major underlying pathophysiology leading to the clinical and imaging picture. Similar clinical and imaging findings can be seen in patients with vein of Galen malformations. This supports our contention that regardless of the specific underlying lesion, common clinical and imaging findings can result if the underlying lesion results in venous hypertension.
References
- Albright AL, Latchaw RE, Price RA: Posterior dural arteriovenous malformations in infancy. Neurosurgery 13: 129-135, 1983
- Awad IA, Little JR, Akrawi WP, et al: Intracranial dural arteriovenous malformations: Factors predisposing to an aggressive neurological course. J Neurosurg 72: 839-850, 1990
- Barnwell SL, Halbach VV, Higashida RT, et al: Complex dural arteriovenous fistulas. Results of combined endovascular and neurosurgical treatment in 16 patients. J Neurosurg 71: 352-358, 1989
- Cataltepe O, Berker M, Gurcay O, et al: An unusual dural arteriovenous fistula in an infant. (Review). Neuroradiology 35: 394-397, 1993
- Cognard C, Gobin YP, Pierot L, et al: Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology 194: 671-680, 1995
- Cronqvist S, Granholm L, Lundström N-R: Hydrocephalus and congestive heart failure caused by intracranial arteriovenous malformations in infants. J Neurosurg 36: 249-254, 1972
- Duckwiler G: Dural arteriovenous fistula. Neuroimaging Clin N Am 2: 291-307, 1992
- Garcia-Monaco R, Lasjaunias P, Berenstein A: Therapeutic management of vein of Galen aneurysmal malformations, in Vinuela F, Halbach VV, Dion JE (eds): Interventional Neuroradiology. Endovascular Therapy of the Central Nervous System. New York: Raven, 1992, pp 113-127
- Gordon IJ, Shah BL, Hardman DR, et al: Giant dural supratentorial arteriovenous malformation. AJR 129: 734-736, 1977
- Haar FL, Miller CA: Hydrocephalus resulting from superior vena cava thrombosis in an infant. Case report. J Neurosurg 42: 597-601, 1975
- Halbach VV, Higashida RT, Hieshima GB, et al: Endovascular therapy of dural fistulas, in Viñuela F, Halbach VV, Dion JE (eds):Interventional Neuroradiology. Endovascular Therapy of the Central Nervous System. New York: Raven, 1992, pp 29-50
- Halbach VV, Higashida RT, Hieshima GB, et al: Treatment of dural fistulas involving the deep cerebral venous system. AJNR 10:393-399, 1989
- Houser OW, Campbell JK, Campbell RJ, et al: Arteriovenous malformation affecting the transverse dural venous sinus—an acquired lesion. Mayo Clin Proc 54:651-661, 1979
- Kinal ME: Hydrocephalus and the dural venous sinuses. J Neurosurg 19:195-201, 1962
- Konishi Y, Hieshima GB, Hara M, et al: Congenital fistula of the dural carotid-cavernous sinus: Case report and review of the literature. Neurosurgery 27:120-126, 1990
- Lamas E, Lobato RD, Esparza J, et al: Dural posterior fossa AVM producing raised sagittal sinus pressure. Case report. JNeurosurg 46:804-810, 1977
- Lasjaunias P, Berenstein A: Dural arteriovenous malformations (DAVM), in Lasjaunias P, Berenstein A (eds): Surgical Neuro-Angiography. Volume 2. Endovascular Treatment of Craniofacial Lesions. Berlin: Springer-Verlag, 1987, pp 274-315
- Lasjaunias P, Berenstein A: Arteriovenous fistulas of the brain, in Lasjaunias P, Berenstein A (eds): Surgical Neuro-Angiography. Volume 4. Endovascular Treatment of Cerebral Lesions. Berlin: Springer-Verlag, 1992, pp 267-317
- Lewis AI, Tomsick TA, Tew JM, Jr: Management of tentorial dural arteriovenous malformations: Transarterial embolization combined with stereotactic radiation or surgery. J Neurosurg 81: 851-859, 1994
- Lownie SP: Intracranial dural arteriovenous fistulas: Endovascular therapy. Neurosurg Clin N Am 5: 449-457, 1994
- Mickle JP, Quisling RG: Vein of Galen fistulas. Neurosurg Clin N Am 5: 529-540, 1994
- Mullan S: Reflections upon the nature and management of intracranial and intraspinal vascular malformations and fistulae. J Neurosurg 80: 606-616, 1994
- Newton TH, Weidner W, Greitz T: Dural arteriovenous malformation in the posterior fossa. Radiology 90: 27-35, 1968
- Obrador S, Soto M, Silvela J: Clinical syndromes of arteriovenous malformations of the transverse-sigmoid sinus. J Neurol Neurosurg Psychiatry 38: 436-451, 1975
- Ross DA, Walker J, Edwards MS: Unusual posterior fossa dural arteriovenous malformation in a neonate: Case report.Neurosurgery 19: 1021-1024, 1986
- Tsugane R, Sato O, Watabe T: Non-communicating hydrocephalus caused by dural arteriovenous malformation. Surg Neurol 12:393-396, 1979
