Metastatic Brain Tumors: Gamma Knife Radiosurgery or Microsurgical Resection?
Kris A. Smith, MD
Division of Neurological Surgery, Barrow Neurological Institute, Mercy Healthcare Arizona, Phoenix, Arizona
Abstract
Metastatic brain tumors are the most common brain tumors in adults. Without treatment, such patients die within 1 or 2 months, and about 50% die directly from the brain metastasis rather than from the primary disease. Radiosurgery has become an accepted alternative to microsurgical resection as treatment for metastatic tumors. Compared to surgical resection, radiosurgery decreases the patient’s discomfort, requires no surgical resection, shortens hospital stays, and lowers treatment costs. Tumors in locations that would have been associated with unacceptable risks for microsurgical resection also can be treated. With radiosurgery, however, tissue is unavailable for pathological analysis, tumors larger than 3 or 4 cm in diameter cannot be treated adequately, and mass effect and edema are reduced more slowly than when tumors are resected. Tumors also recur more frequently after radiosurgical treatment than when tumors are removed surgically. Surgical series of selected patients show more long-term survivors than those reported thus far in radiosurgical series. This article reviews and analyzes prospective and retrospective studies of radiosurgical and microsurgical treatment of brain metastasis. The shortcomings of existing studies indicate the need for a randomized prospective trial that compares radiosurgery to microsurgery for the treatment of patients with metastatic brain tumors.
Key Words : brain tumors, frameless stereotaxy, Gamma Knife, metastasis, microsurgery, radiosurgery
Stereotactic radiosurgery, defined as “the delivery of a single, high dose of radiation to a small and critically located intracranial volume,” by its developer, Lars Leksell, has now become an attractive alternative to microsurgical resection for brain metastasis. Compared to microsurgical resection, radiosurgery has several advantages. First, radiosurgery is a noninvasive treatment that requires only the placement of a stereotactic frame with local anesthetic and sedation. Second, radiosurgery avoids the risks of general anesthesia and immediate perioperative risks such as bleeding, cerebrospinal fluid leaks, or infections. Finally, radiosurgery is associated with lower treatment costs and briefer hospital stays compared to conventional surgery.14 Consequently, radiosurgery—either with the Gamma Knife or with a modified linear accelerator—has become widely used to treat brain metastasis. In the most recent update by the Leksell Gamma Knife Society, with all cases reported as of June 30, 1996, metastatic brain tumor was the most common indication for treatment with the Gamma Knife. Of 49,143 treatments given with the Gamma Knife since its development in 1968, 9,349 of those indications have been for metastatic brain tumors. Of those, 2,653 have been treated in the United States. This year in the United States approximately 1000 patients will be treated for metastatic brain tumors with the Gamma Knife.
Natural History and Treatment Outcomes
Cancer is the second leading cause of death in the United States.15 Autopsy series of patients who died from cancer document intracranial metastatic disease in 10 to 50% of the cases.6,13,15,20 Each year in the United States, there are an estimated 100,000 to 150,000 new cases of brain metastasis and the incidence may be increasing due to longer survival times from improved treatments of the primary disease sites.16,21 Also, the incidence of melanoma, which has a predilection for metastasis to the brain, is increasing.16
Although metastasis to the brain usually occurs late in the stage of illness when other areas of the body have widespread systemic disease, symptomatic brain metastasis is the first sign of cancer in many patients.1,4,16,18 Metastatic brain cancer with an unknown primary site, even after extensive work-up, is common, comprising up to 11% of the cases in neurosurgical series of resected metastasis.3,4,6,18,21 With magnetic resonance (MR) imaging as the preferred screening modality for detection of metastatic disease to the brain, the incidence of neurologically asymptomatic patients with documented intracranial metastasis will no doubt increase.
The natural history of brain metastasis has a dismal prognosis with a median survival of 1.2 months from diagnosis without treatment.4,5,21 In 50% of the patients with intracerebral metastasis, the immediate or directly contributing cause of death is the brain metastasis itself rather than the systemic disease.21 Treatment with corticosteroids increases survival to 2 months, and the traditional treatment of fractionated WBRT increases the median survival to 4.5 months.21 The addition of focally aggressive treatment, either radiosurgery or microsurgical resection of the metastasis, increases the median survival to about 12 months.2-4,7,10-13,16,18,19,21 The median survival of selected patients with otherwise well-controlled systemic disease and a solitary brain metastasis treated with surgical resection combined with WBRT increases to 16 months and as many as 21% survive longer than 5 years.3,4,16,21
Surgical Resection of Brain Metastasis
Many retrospective series have found a meaningful improvement in quality of life and prolonged survival after the surgical resection of a solitary brain metastasis in selected patients.1,3,4,10,11,16,18 Two randomized, prospective trials also have documented improved survival and longer functional independence in patients undergoing microsurgical resection of a solitary metastasis combined with WBRT compared to patients undergoing WBRT alone.13,19 Nevertheless, some oncologists and non-neurosurgical caregivers have been reluctant to recommend such an aggressive treatment approach for many cancer patients with metastatic disease to the brain.
In 1990 Patchell et al.13 reported the first randomized prospective trial comparing the results of surgical resection of a single brain metastasis coupled with WBRT compared to those of WBRT alone. Preoperative MR imaging documented only one brain metastasis in 48 consecutive patients, all of whom also had pathological confirmation of their metastatic disease (by stereotactic biopsy in the WBRT group). The pretreatment Karnofsky scores of all patients were greater than 70%. Extent of systemic disease was not an exclusion criterion. This criterion was paramount to insure that criticisms aimed at earlier series were addressed. These studies had excluded patients with systemic disease from their surgical cohorts, thereby biasing survival times in their favor. In the Patchell study, patients were stratified according to primary histology and the presence or absence of metastatic disease other than in the brain. Brain tumor location (supratentorial vs. infratentorial) was evenly distributed between the two groups. All patients received fractionated WBRT (36 Gy), and all were followed with serial computed tomography or MR imaging to assess local control of the metastasis. Serial Karnofsky scores were used to measure quality of life. No patients were lost to follow-up and the cause of death was determined in all patients who died.
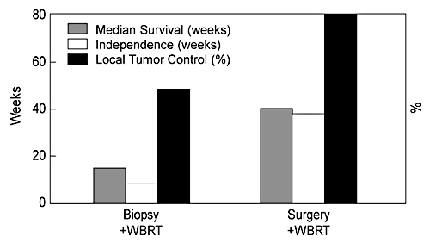
The median survival time, median length of independence (weeks of Karnofsky scores greater than 70%), and the percent of local control were statistically significant in favor of the surgical group (Fig. 2). In both groups, the 30-day mortality rate was 4%. This well-designed, prospective study conclusively demonstrated a significant benefit from a combination of surgical resection and WBRT in cancer patients with a single brain metastasis. Because this study included patients with significant systemic disease, the median survival of 40 weeks was not as long as that reported in other surgical series in which the majority of patients had controlled systemic disease. Clearly, patients who died from their systemic disease within 2 months of surgery did not benefit from the treatment. The study concludes, however, that patients with a surgically accessible single brain metastasis, Karnofsky scores greater than 70%, and a life expectancy from their systemic disease longer than 2 months should be considered for surgical resection.
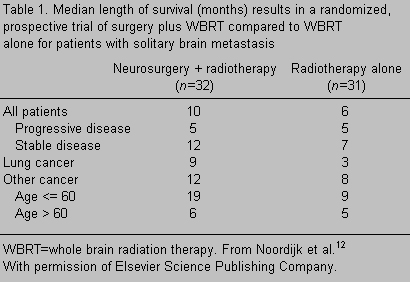
In 1993 another prospective randomized trial19compared treatment with fractionated WBRT alone to surgical resection plus WBRT in 63 patients. The extent of active systemic disease and various primary tumor types were evenly and prospectively stratified between the two groups. The results further supported the findings of the Patchell study13 (Table 1). The median survival for the surgery plus WBRT group was 10 months compared to 6 months for the group treated with WBRT alone (p = 0.04). However, patients with active systemic disease did not benefit from surgery since the median survival of those patients in both groups was 5 months. The benefit of surgery was more apparent in patients with controlled systemic disease. In this subset of patients, the median survival of the surgical arm was 12 months compared to 7 months in the WBRT arm (p = 0.02). The 2-year survival rate was 30% in the former compared to 10% in the latter. The only two long-term survivors (34 and 78 months, respectively) who were still alive at the time of publication were in the surgical arm.
A later analysis of this study found a marked difference in survival as a function of age.12 The median length of survival of patients younger than 60 years in the surgical arm was 19 months compared to 9 months in the WBRT arm. In patients older than 60, however, the addition of surgery only increased their median survival from 5 to 6 months. Still, most patients in the surgical arm were functionally independent longer than those in the WBRT arm and remained so until shortly before dying of their systemic disease. The authors concluded that surgical resection of solitary brain metastasis plus WBRT should be offered to cancer patients with controlled systemic disease, especially those younger than 60.
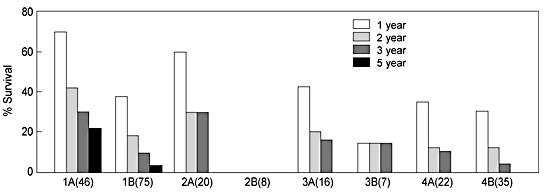
In 1992, Smalley et al.16 reported a retrospective study of 229 consecutive patients treated surgically for a solitary brain metastasis. By multivariate analysis, the authors attempted to determine which factors predicted survival and a clear benefit from surgical resection of metastatic brain disease at 1, 2, 3, and 5 years in different subgroups of patients (Fig. 3). The addition of WBRT to resection significantly increased survival compared to surgical excision alone. The most favorable subgroup was group 1A, which had no systemic disease and in whom a gross total resection of the metastatic tumor was accomplished in addition to WBRT. Their median survival was 16 months and their 5-year survival rate was 21%. This study further emphasizes the importance of controlled systemic disease and the absence of a severe neurological deficit at the time of resection. Survival also improved if the interval between the diagnosis of the primary cancer and the detection of the brain metastasis was longer than 36 months. Most of the patients in all of the groups had lung cancer. Overall, patients with lung cancer did less well than patients with other types of cancer (e.g., breast cancer). However, there were long-term survivors with lung cancer in this series.
As with all retrospective studies, the possibility of a selection bias must be considered when the results of this study are interpreted and conclusions drawn. For example, the dose and protocol of radiation therapy varied widely among the patients. And it is unclear why patients who did not receive WBRT were treated in this manner. If they were selected to not receive WBRT because no benefits were expected from the therapy, then the conclusions of the study are meaningless. However, the large number of patients in this series and the extensive and thoughtful multivariate analysis used to address the possibility of bias suggest that the conclusions are likely valid.
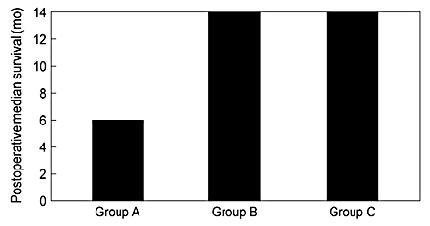
In 1993 Bindal et al.4 retrospectively reviewed 56 patients to evaluate whether the palliation associated with the removal of multiple metastases would be as favorable as that achieved with the removal of a single metastasis. Group A consisted of 30 patients from whom 40 lesions were resected but who still harbored other lesions after surgery. All known lesions were removed in Group B, 26 patients from whom 55 lesions were resected via 44 craniotomies. Group C was composed of patients who had a single brain metastasis, which was removed. The median survival of Group A was only 6 months compared to 14 months in the other two groups (Fig. 4). This study supports the notion that the surgical removal of two or more metastases may result in meaningful palliation for select patients.
There are, however, problems with this study. Age, sex, Karnofsky score, primary tumor histology, and percent of patients with systemic disease (59%) were all evenly distributed. However, tumor location was not mentioned. Certainly, the patients in Group A were selected because the unresected lesions were difficult to access surgically. The major question raised by this study is whether radiosurgical treatment of surgically inaccessible lesions would confer as much benefit as it does to those in whom all known lesions are removed. The question of “how many lesions are too many?” also arises but is not addressed by this study in which most of the patients with multiple metastases really had only two metastases, at least in the group that underwent complete resection of all lesions.
Radiosurgery for Brain Metastasis
In 1994 Flickinger et al.7 reported the experience of Gamma Knife radiosurgery for solitary brain metastasis from five centers in the United States (n = 116); the median follow-up was only 7 months from treatment. As in many retrospective multi-institutional studies, patient selection, dose (range, 8 to 30 Gy), and adjuvant treatments, such as fractionated radiation and chemotherapy protocols, varied widely if reported at all. The types of primary tumors also varied. Lung cancer was the most common diagnosis (35%), followed by malignant melanoma (24%), renal cell carcinoma (12%), and breast cancer (11%).
The actuarial median survival of the whole group after radiosurgery was 11 months. Local tumor control of the treated metastasis was 85%; 15% of the tumors continued to grow or recurred. Interestingly, melanoma and renal cell carcinoma, which typically respond poorly to traditional fractionated radiation therapy, showed an excellent response (100% local control). However, the median survival of these patients was only 8 months. A multivariate analysis was performed to identify factors that improved prognosis. Combined WBRT with radiosurgery correlated with a longer survival than radiosurgery alone. A younger age and the diagnosis of breast cancer were also associated with prolonged survival. This study supports radiosurgery as an adjunct to WBRT in the treatment of cancer patients with a solitary brain metastasis. However, its retrospective nature precludes comparison with the randomized surgical studies discussed above because patient selection and treatment were not standardized.
In 1994, Jokura et al.9 reported 25 consecutive, “minimally selected” metastatic brain tumor patients who underwent Gamma Knife treatment. These patients had “surgically inaccessible” lesions or had refused open surgery. Altogether, 77 lesions were treated (one patient had 16 tumors). WBRT was given to only 3 of the 25 patients and radiosurgery was the only treatment given to the remaining 22. The mean dose delivery to the tumor margin was 26.5 Gy.
The median survival for the whole group was 8.5 months: 10.5 months for those with a single metastasis but only 2.5 months for those with multiple metastases. Two patients died within one month of treatment. According to their Karnofsky scores, the other 23 improved neurologically (n = 11) or stabilized (n = 12). Only four patients were said to have died from their central nervous system (CNS) disease. The results of this study are somewhat anecdotal since the extent of the patients’ systemic disease is not mentioned. However, the 10.5-month survival for patients with a single metastasis is at least comparable to the results of the surgical series and further supports radiosurgery as a treatment alternative for patients with surgically inaccessible lesions. It is also difficult to know if WBRT would have made any difference to the outcome of this study. Certainly, there is a point (i.e., 16 metastases) when treatment is unwarranted.
Radiosurgery versus Surgical Resection
In 1996, Auchter et al.2 reported a group of patients retrospectively selected from the databases of four institutions that use modified linear accelerators for the radiosurgical treatment of brain metastasis. An optimal group of 122 patients was selected from 533 patients with brain metastasis treated at the four institutions. These 122 patients were selected based on the following: a single metastasis in a surgically accessible location, a Karnofsky score greater than 70%, age, gender, and status of primary disease (only 9% were stated to have active systemic disease). All but five patients also received WBRT under variable protocols. The median peripheral radiosurgical dose was 17 Gy. The median follow-up was 123 weeks
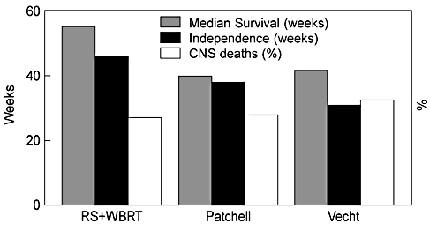
The actuarial median survival of the 122 patients was 56 weeks with 1- and 2-year survival rates of 53 and 30%, respectively. The median duration of functional independence was 44 weeks. Of the 77 deaths, 19 (25%) were attributable to CNS disease. There were 27 (22%) new tumors outside the treatment volume. The local control rate was 86% since 14% of the patients had continued growth of their tumors after treatment. However, only 59% of the patients experienced actual resolution of their tumors (25%) or more than 50% reduction in its size (34%). The results of surgical treatment for solitary metastasis were compared to the results of the surgery plus WBRT groups of both prospective randomized trials cited above (Fig. 5).2,13,19 This comparison, however, is invalid because a clear selection bias exists. In the prospective studies, the surgically treated group included more patients with active systemic disease. It is unfair to compare a retrospectively selected group of optimal patients to a prospectively randomized group when the randomization includes patients with systemic disease. This study supports the usefulness of radiosurgery for selected patients with metastatic brain tumors. It does not, however, demonstrate the superiority, or even the equivalency, of radiosurgery to surgical resection.
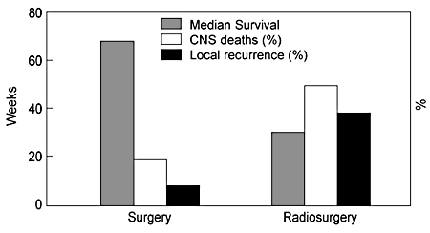
In 1996 Bindal et al.3 retrospectively compared surgically and radiosurgically treated patients with brain metastasis at one institution over the same time period. Thirty-one consecutive patients treated by radiosurgery (modified linear accelerator; peripheral dose range, 17 to 22 Gy) were retrospectively matched to 62 patients treated surgically (from 500 surgical patients). The groups were matched for histology, age, sex, time to metastasis, Karnofsky score, and extent of systemic disease. The patients were not matched for tumor size or location or for treatment with WBRT. In the radiosurgery group, “80.5% of the tumors were surgically resectable with minimal morbidity.” The results of this comparison markedly favored the surgical group (Fig. 6), which had a median survival of 16.4 months compared to 7.5 months for the radiosurgery group. Local control also was superior in the surgical group. Only 5 patients (8%) from the surgical group had a local recurrence compared to 12 patients (39%) whose tumor progressed after radiosurgery.
This retrospective comparison may also suffer from a selection bias. Why, for example, were patients who were treated at the same institution and who were not randomized, referred for an alternate therapy? If, for example, the 20% of the tumors in the radiosurgical arm that were not in surgically resectable locations were in the midbrain or brain stem, these patients would have had a poor prognosis and would have been likely to die of their neurological disease. Even without the possibility of this selection bias, the lack of local control in the radiosurgical group, which may relate to the dosage protocol used in this series, is of concern. The peripheral dose range of 17 to 22 Gy is similar to other series. However, the maximum tumor dose was only 22 Gy (i.e., 100% isodose curve at the tumor periphery), which is much less than reported in other Gamma Knife series (34 to 50 Gy maximum with the 50% isodose curve at the periphery). Still, the differential effect of the dosage protocol is only speculation. No comparative study of these two techniques and dosing protocols for metastatic tumors has been done to confirm that a difference in local tumor control is related to dosing protocol.
In 1995 Rutigliano et al.14 compared the cost effectiveness of radiosurgical and surgical treatments for brain metastasis. The analysis was thoughtful and extensive, including hidden costs of capital, administration, treatment, complications, and follow-up. Cost effectiveness as measured by “cost per life-year added” clearly favored radiosurgery. The major flaw of the analysis, however, is that the costs of the surgical group and data regarding length of hospitalization were primarily derived from patients operated on in the 1980s. The prices were then inflated to 1990s prices to enable the comparison with contemporary patients in the radiosurgical group reported by Flickinger et al.7 In this day of cost-consciousness, the authors note that any prospective comparisons of two alternative treatments should include the costs of therapy.
Discussion
The optimal management of patients with metastatic brain disease poses many difficult questions for patients, families, caregivers, payors and society as a whole. Issues related to quality of life as well as the cost of palliative treatment in terminal illness are valid concerns when treatment options for patients with brain metastasis are being considered. When confronted with an individual patient with a metastatic brain tumor, however, statistical analysis and treatment costs mean little to those trying to offer individuals the best possible treatment.
This review suggests that meaningful palliation can be achieved for many patients with metastatic disease to the brain. Furthermore, in some cases, long-term survival is possible when aggressive focal treatment is combined with WBRT. Patient selection for treatment is always easier in retrospect. However, younger patients with a single or possibly two or three metastases to the brain without progressive systemic disease appear to benefit from either surgery or radiosurgery plus WBRT. Survival and, more importantly, functional independence are prolonged with either focal treatment. It now becomes necessary to weigh the advantages and disadvantages of both treatments to decide which treatment is best for which patients. If a clear-cut advantage cannot be established for certain groups of patients, then a randomized prospective trial is necessary to establish medically and ethically sound recommendations.
In many cases, neurosurgical resection must still be considered as the gold standard of focal therapy. Its benefit compared to WBRT alone has been established in randomized prospective trials. In select patient groups with ideal prognostic indicators, such as age less than 60 years, a Karnofsky score greater than 70%, a single metastasis in accessible location, and no systemic disease, the median survival is as long as 16 months and as many as 21% of the patients survive 5 years.3,16 To date, such favorable outcomes have not been duplicated by radiosurgery. Surgical resection also immediately eliminates the mass effect from large, symptomatic tumors. Modern neurosurgical techniques, with frameless stereotactic guidance, may even increase the percentage of complete resections that is possible. Because intraoperative guidance permits the size of craniotomies to be minimized, morbidity rates, patient discomfort, length of hospitalization, and therefore costs may all decrease. If so, the advantages in terms of cost and comfort would be narrowed between radiosurgery and resection. Currently, however, open surgery to resect small deeply located lesions risks considerable neurological morbidity.
Radiosurgery is essentially an outpatient procedure performed under local anesthetic, and there is little risk associated with the initial treatment. While radiosurgery appears to be less expensive than microsurgery, no contemporary cost comparisons have been done. Such a study would also need to consider the cost of the additional biopsy needed in some patients to confirm the metastasis. Radiosurgery is less limited by lesion location than open surgery, and treating more than one lesion in one sitting is not technically difficult. The technology also has improved since its development. The computer programming for treatment planning has been updated continuously and permits more accurate conformational dose planning that may increase the efficacy and safety of the technique compared to historical series.
Patient selection biases must always be considered when older microsurgical series are compared to contemporary radiosurgical series. If healthier patients with accessible lesions were selected for surgery and sicker patients with more central lesions were referred for radiosurgery, then the median survival of patients would be expected to be less in the latter group compared to the former. Variations in radiosurgical technique and dosing protocols may also affect outcomes. The efficacy of radiosurgery is dose-dependent, and the dose delivered to the central tumor may be only half that delivered in another series even though the peripheral tumor doses are the same. The efficacy of radiosurgery also is limited by the size of the tumor since the dose must be decreased as the tumor volume increases to avoid the increased risk of radiation damage to the surrounding normal brain tissue. An appropriate treatment dose needs to be established and incorporated into a prospective trial to eliminate this important variable as much as possible in further comparisons of treatments.
Conclusion
Depending on certain characteristics of the tumor and patient, both surgical resection and radiosurgery have clear advantages for the treatment of brain metastasis. For example, superficial lesions greater than 3 cm with marked mass effect and edema should be treated surgically. Alternatively, smaller lesions in central locations or most lesions in patients with increased surgical risk factors should be treated with radiosurgery. However, neither treatment has a clear advantage in the remaining large group of patients. The retrospective studies discussed above suffer from multiple flaws that permit authors to draw conclusions according to their particular bias. The popularity of radiosurgery is increasing, and a large numbers of patients will be treated with this technique in the future. Therefore, this treatment must be compared to the gold standard of microsurgical resection in a randomized prospective trial. For the comparison to be valid, patients in such a trial must be well matched and subjected to clearly defined inclusion criteria that include status of systemic disease, general health, surgical accessibility of the tumor, and a standardized radiosurgical dosing protocol. Ideally, the comprehensive cost of treatment would also be collected prospectively so that an accurate contemporary comparison would be available. A contemporary, prospective, randomized trial, void of selection biases, is crucial to establishing the superiority, if any, of one treatment over the other.
References
- Arbit E, Wronski M, Burt M, et al: The treatment of patients with recurrent brain metastasis. A retrospective analysis of 109 patients with nonsmall cell lung cancer. Cancer 76:765-773, 1995
- Autcher RM, Lamond JP, Alexander E, III, et al: A multiinstitutional outcome and prognostic factor analysis of radiosurgery for resectable single brain metastasis. Int J Radiat Oncol Biol Phys 35:27-35, 1996
- Bindal AK, Bindal RK, Hess KR, et al: Surgery versus radiosurgery in the treatment of brain metastasis. J Neurosurg 84:748-754, 1990
- Bindal RK, Sawaya R, Leavens ME, et al: Surgical treatment of multiple brain metastases. J Neurosurg 79:210-216, 1993
- De Salles AAF, Hariz M, Bajada CL, et al: Comparison between radiosurgery and stereotactic fractionated radiation for the treatment of brain metastases. Acta Neurochir (Wien) 58:115-118, 1993
- Delattre JY, Krol G, Thaler HT, et al: Distribution of brain metastases. Arch Neurol 45:741-744, 1988
- Flickinger JC, Kondziolka D, Lunsford LD, et al: A multi-institutional experience with stereotactic radiosurgery for solitary brain metastasis. Int J Radiat Oncol Biol Phys 28:797-802, 1994
- Gill SS: Radiosurgery for brain tumours: Is there a proven benefit? Br J Neurosurg 9:123-125, 1995
- Jokura H, Takahashi K, Kayama T, et al: Gamma Knife radiosurgery of a series of only minimally selected metastatic brain tumours.Acta Neurochir (Suppl) 62:77-82, 1994
- Mandell L, Hilaris B, Sullivan M, et al: The treatment of single brain metastasis from non-oat cell lung carcinoma. Surgery and radiation versus radiation therapy alone. Cancer 58:641-649, 1986
- Nakagawa H, Miyawaki Y, Fujita T, et al: Surgical treatment of brain metastases of lung cancer: Retrospective analysis of 89 cases.J Neurol Neurosurg Psychiatry 57:950-956, 1994
- Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al: The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys 29:711-717, 1994
- Patchell RA, Tibbs PA, Walsh JW, et al: A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 322:494-500, 1990
- Rutigliano MJ, Lunsford LD, Kondziolka D, et al: The cost effectiveness of stereotactic radiosurgery versus surgical resection in the treatment of solitary metastatic brain tumors. Neurosurgery 37:445-455, 1995
- Silverberg E, Lubera JA: Cancer statistics, 1988. CA Cancer J Clin 38:5-22, 1988
- Smalley SR, Laws ER, Jr., O’Fallon JR, et al: Resection for solitary brain metastasis. Role of adjuvant radiation and prognostic variables in 229 patients. J Neurosurg 77:531-540, 1992
- Somaza S, Kondziolka D, Lunsford LD, et al: Stereotactic radiosurgery for cerebral metastatic melanoma. J Neurosurg 79:661-666, 1993
- Sundaresan N, Galicich JH, Beattie EJ, Jr: Surgical treatment of brain metastases from lung cancer. J Neurosurg 58:666-671, 1983
- Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al: Treatment of single brain metastasis: Radiotherapy alone or combined with neurosurgery. Ann Neurol 33:583-590, 1993
- Walker AE, Robins M, Weinfeld FD: Epidemiology of brain tumors: The national survey of intracranial neoplasms. Neurology 35:219-226, 1985
- Zimm S, Wampler GL, Stablein D, et al: Intracerebral metastases in solid-tumor patients: Natural history and results of treatment.Cancer 48:384-394, 1981