
Surgical Management of Intracavernous Carotid Artery Aneurysms: BNI Experience
Authors
A. Giancarlo Vishteh, MD
Suresh K. Sankhla, MD†
Michael T. Lawton, MD
Robert F. Spetzler, MD
Division of Neurological Surgery, Barrow Neurological Institute, Mercy Healthcare Arizona, Phoenix, Arizona
†Current Address: Department of Neurosurgery, University of Texas, M. D. Anderson Cancer Center, Houston, Texas
Abstract
Between 1988 and 1994, 24 patients (21 females and 3 males) with 25 aneurysms of the cavernous portion of the internal carotid artery (ICA) underwent surgery. Patients presented with signs and symptoms of cranial nerve compression (n=15), subarachnoid hemorrhage (n=3), thromboembolic ischemic symptoms (n=3), bilateral carotid-cavernous fistulae (n=1), and seizures (n=1). One aneurysm was discovered incidentally. Eighteen aneurysms were confined to the cavernous sinus, while seven had subarachnoid extensions. There were 15 giant and 9 large aneurysms and 1 small aneurysm. Surgical intervention included trapping and bypassing (n=17), direct clipping (n=5), or wrapping (n=3). At a mean follow-up of 2.0 years, 22 (92%) patients were at their preoperative Glasgow Outcome Scale score or better. Two patients (8%) had deteriorated. There were no deaths related to surgery, and the treatment-associated neurological morbidity rate was 8%. Aneurysms of the cavernous ICA have become amenable to surgery, with good patient outcomes, mainly due to advances in microsurgical, vascular bypass, and endovascular techniques. Surgery, however, is recommended only for a few select cases with clear and appropriate indications.
Key Words : aneurysms, carotid artery, cavernous sinus, revascularization
Intracavernous internal carotid artery (ICA) aneurysms represent 3 to 5% of all intracranial aneurysms16,35,49 and account for 14% of all ICA aneurysms.47 As neuroimaging techniques have become more sophisticated, the diagnosis of cavernous aneurysms has increased markedly. About a third of these lesions are asymptomatic at diagnosis. Their natural history suggests that a large number of such aneurysms will remain clinically asymptomatic31 and carry significantly lower rates of rupture and mortality compared to aneurysms situated in the subarachnoid compartment.27,32 Therefore, most asymptomatic intracavernous ICA aneurysms do not require surgical treatment. Because the morbidity and mortality rates associated with surgical treatment are high, surgery should be reserved for cases with clear indications.7-9,18,37,40,41,43-46,50 For example, asymptomatic aneurysms that extend into the subarachnoid space or those located at the junction of the cavernous and intradural portions of the ICA are associated with a considerable risk of subarachnoid hemorrhage (SAH) and should be considered for elective surgical management.1-33
Symptomatic lesions may produce considerable neurological morbidity as the result of compression (pain and cranial nerve deficits), rupture (carotid-cavernous fistula, epi staxis or SAH), or thromboembolic episodes associated with ischemia.3,14,31,38,58 Patients with symptomatic aneurysms, particularly those who present with acute rupture or progressive neurological manifestations, may incur profound debilitating or potentially life-threatening consequences from their disease and hence require treatment. Therefore, direct surgical treatment (combined with endovascular intervention in some cases) is one approach to the management of patients with symptomatic intracavernous ICA aneurysms.9,10 We present our experience with the surgical treatment of aneurysms of the intracavernous ICA in 24 patients. Clinical, anatomical, and surgical features are discussed. Based on our experience and review of the literature, we propose a treatment protocol for intracavernous ICA aneurysms.48
Clinical Materials and Methods
Patient Population
Between May 1988 and April 1994, 24 patients with aneurysms arising from the intracavernous portion of the ICA were treated surgically at our institution (Table 1). There were 21 females and 3 males (mean age, 52 years; range, 6 to 78 years). Aneurysms originating outside the cavernous sinus but with part of their sac extending into the cavernous sinus as well as paraclinoid aneurysms that required entry into the cavernous sinus at surgery were excluded. Hospital and office records were examined for clinical data and radiological studies for anatomic features. All surgical procedures were performed by one surgeon (RFS). Pre- and postoperative neurological function was evaluated using Glasgow Outcome Scale (GOS) scores.28 Final outcome was assessed during clinic visits as well as by telephone questionnaires.
Clinical Presentation
Fifteen (63%) patients presented with signs and symptoms of progressive compression of cranial nerves (II through VI) as a result of aneurysmal enlargement (Table 1 ). Three (13%) patients presented with SAH, and all were Hunt and Hess26 grade II at diagnosis. Three (13%) patients had thromboembolic symptoms and one (4%) patient became symptomatic with seizures. One (4%) patient presented with bilateral carotid-cavernous fistulae after trauma, and one (4%) patient’s aneurysm was detected incidentally during investigations for unrelated symptoms that included mild diaphoresis, nausea and confusion. By preoperative GOS criteria, 19 patients were in good condition and 5 had moderate disabilities. Twenty patients had preoperative neurological deficits.
Radiographic Features
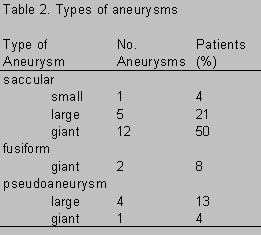
Cerebral angiography, the gold standard for determination of the origin, configuration, and course of an aneurysm, was performed in all patients. Radiological studies demonstrated 38 intracranial aneurysms, 29 of which were located within the cavernous sinus. Of these 29 intracavernous ICA aneurysms, 25 were treated surgically and therefore included in this series. Eighteen (72%) aneurysms were confined to the cavernous sinus only; the remaining seven (28%) aneurysms extended into the adjacent subarachnoid space. There was a predominance of right-sided aneurysms (16:9). There were 15 (60%) giant (>2.5 cm in diameter) and 9 (36%) large (1.0 to 2.5 cm in diameter) aneurysms and one (4%) small (<1.0 cm in diameter) aneurysm (Table 2). Eighteen (72%) aneurysms were classified as saccular, two (8%) as fusiform, and five (20%) as pseudo aneurysms (Table 2).
Surgical Technique
All surgical procedures were performed under standard general anesthesia, including barbiturates. In all cases the function of the cavernous sinus cranial nerves (intraoperative electromyography) and cerebral hemispheres (somatosensory evoked potentials) was monitored during surgery. Proximal control of the carotid artery was established via direct exposure of the vessel in the neck or with the aid of an endovascular catheter with an inflatable balloon placed in the cervical or petrous portion of the ICA. The operations were performed through a standard pterional craniotomy (n = 13) or a combined orbitozygomatic-pterional craniotomy (n = 12). Patients were treated by clipping, trapping with bypass, or other procedures (i.e., wrapping or proximal occlusion).
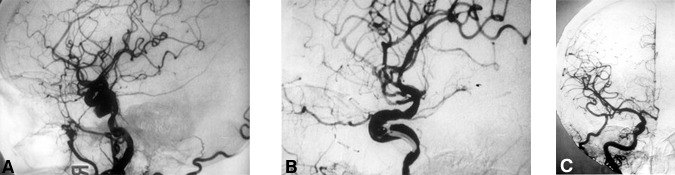
Clipping. Five (21%) patients underwent direct clipping of their intracavernous ICA aneurysms (Table 1, Fig. 1). Four of these patients presented with a progressive cranial nerve compressive syndrome and one presented with SAH. In two patients the aneurysms had subarachnoid extensions.
Aneurysms were clipped through a standard pterional/orbitozygomatic craniotomy using combined epi- and subdural techniques as described elsewhere.9,10 The lateral portion of the lesser wing of the sphenoid and part of the orbital roof and lateral wall were removed extradurally. The dura was opened in the usual manner with its base directed pterionally. The Sylvian fissure was opened widely, and the optic nerve and ICA were dissected free of arachnoid coverings. The dura over the anterior clinoid process was incised subdurally, and the underlying bone was removed using a high-speed diamond drill. The anterior clinoid process and adjacent bony roof of the optic foramen were removed to facilitate exposure of the intracavernous ICA and optic nerve. The dural sleeve covering the optic nerve was then incised to free the nerve.
The cavernous sinus was entered through its roof. The ICA was identified and dissected free of its dural attachments by transecting the distal dural ring, which surrounds the ICA as it enters the subarachnoid space. The normal anatomical relationship of the ICA to the cranial nerves (i.e., oculomotor, trochlear, abducent and the ophthalmic division of the tri geminal) was preserved. The dissection continued along the course of the ICA exposing it within the cavernous sinus until the neck of the aneurysm (if present) was approached. The cavernous sinus was packed with Surgicel to control bleeding during dissection of the intracavernous course of the ICA. The neck of the aneurysm (when present) was dissected and clipped using the operating microscope. Temporary occlusion of the ICA distally in the subarachnoid space as well as proximally, either in the cervical or petrous regions (cases with a C3-C5 bypass), facilitated safer manipulation of the aneurysm during dissection and application of the clip. In one patient (Case 5), hypothermic circulatory arrest and barbiturate-induced coma were used to clip and debulk a giant aneurysm that was causing severe mass effect.
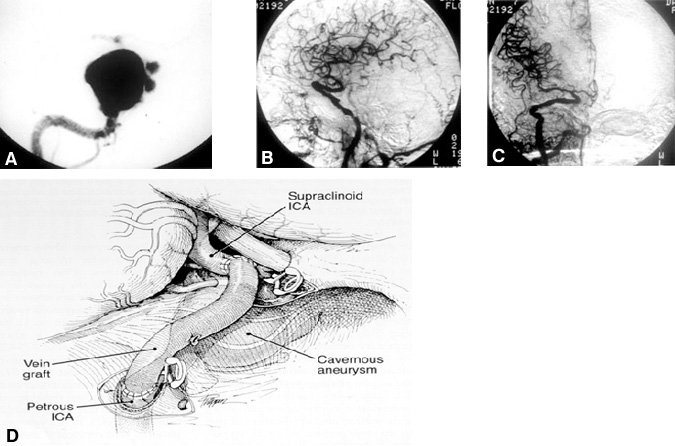
Trapping. Sixteen (64%) aneurysms in 15 patients (Table 1 , Fig. 2) were treated by trapping the ICA. After cerebral revascularization in the same operative setting, the ICA was occluded in the petrous region or neck as well as intracranially immediately distal to the aneurysm. Seven of the patients who underwent trapping had symptoms related to mass effect and compression of the cranial nerves. Two patients presented with SAH, two with cerebrovascular accidents (CVA/TIA), two patients with pseudoaneurysms (three aneurysms altogether) after trauma, and one with seizures. One patient was asymptomatic on presentation. In four patients, the aneurysm extended into the subarachnoid space.
Twelve aneurysms in 11 patients were treated with a standard petrous-to-supraclinoid (C5-C3) ICA bypass using a saphenous-vein graft (Fig. 2). One patient (Case 24), who had bilateral pseudoaneurysms and carotid-cavernous fistulae, underwent bilateral trapping in addition to bilateral C5-C3 ICA bypasses. Two patients had cervical ICA-to-middle cerebral artery (MCA) bypasses using saphenous-vein grafts. One of these patients (Case 21) could not undergo a C5-C3 bypass because the ICA was dolichoectactic from the cavernous to the petrous segments. In another patient (Case 22), an ICA-MCA bypass was prophylactic for an asymptomatic occlusion of the ICA contralateral to the aneurysm. The contralateral bypass was followed by trapping of the aneurysm with an ipsilateral C5-C3 saphenous-vein bypass. Two patients required a superficial temporal artery-(STA)-to-MCA bypass. One of these patients (Case 9) was asymptomatic at presentation and had a giant right intracavernous ICA aneurysm with subarachnoid extension in addition to an ipsilateral ophthalmic artery aneurysm. Failure to clip the giant intracavernous aneurysm intraoperatively led to the decision to trap the aneurysm after an STA-MCA by pass. In one patient (Case 19), the cervical ICA was used for a cervical-to-supraclinoid ICA saphenous-vein bypass because the wall of the petrous portion of the ICA was extremely thin and fragile. In yet another patient (Case 20), a cervical ICA-to-MCA bypass was used because a carotid-cavernous fistula precluded a C5-C3 bypass.
Other Procedures. Four patients received a treatment other than direct clipping of the aneurysm or trapping and bypass. Three patients had compressive signs and symptoms, and their aneurysms were confined to the cavernous sinus. One patient, who had a small cavernous aneurysm with subarachnoid extension, developed transient ischemic attacks localized to the ipsilateral hemisphere.
Three (12%) patients underwent wrapping of their aneurysms for reinforcement. Direct clipping had initially been planned, but technical limitations necessitated wrapping instead. The fourth patient’s (Case 23) intracavernous ICA aneurysm was found to be unclippable during surgery, and she was treated with proximal occlusion of the ICA and an STA-MCA bypass instead. The ICA was occluded in the cervical and cavernous regions using detachable endovascular balloons.
Results
Complications
Nine (38%) patients suffered postoperative complications (Table 1), including early graft occlusion (n = 2). One of the two patients (Case 17) with a graft occlusion underwent immediate reopening of the graft with endovascular tissue plasminogen activator, but her neurological status deteriorated. The other patient with a graft occlusion (Case 20) underwent open revision of the graft successfully, and the patient’s symptoms were stabilized to her preoperative baseline.
Other complications included postoperative epidural hematomas (n = 2) and one case each of transient oculomotor nerve paresis and hearing impairment. One patient (Case 21) developed hemiparesis, vocal cord paresis, Herpes encephalitis, and pneumonia. One patient (Case 9) with a postoperative myocardial infarction deteriorated clinically and showed little improvement on follow-up. Another patient’s (Case 3) aneurysm clip slipped and an additional surgical procedure was required to reposition it. All of these patients had recovered completely at follow-up except for the patient with the myocardial infarction.
Outcomes
At a mean follow-up of 2.0 years, 22 (92%) patients had recovered to their preoperative GOS score or better, and two (8%) patients had deteriorated clinically (Table 1). There were no deaths related to surgery, and the treatment-associated neurological morbidity rate was 8%.
Postoperatively, the neurological condition of all five patients who underwent clipping had improved. Two of these patients were completely asymptomatic at their follow-up examination (Table 1). Of the 15 patients who underwent trapping, 13 (87%) improved and 2 (13%) deteriorated after surgery. Symptoms and neurological deficits also improved in the four patients who underwent procedures other than clipping or trapping. One patient (Case 7) was completely asymptomatic at follow-up.
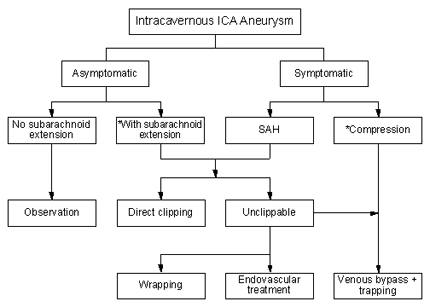
In accordance with our treatment protocol (Fig. 3), direct clipping was attempted in all seven (29%) patients whose intracavernous ICA aneurysm extended into the subarachnoid space. Clipping, however, was successful in only two of these seven patients. The aneurysms of the other five patients were deemed unclippable during surgery: four underwent trapping and one patient underwent wrapping. All but one of these patients (Case 9) experienced a good postoperative neurological recovery, and five were completely normal at follow-up.
Discussion
With advances in radiological and microsurgical techniques over the past few years, the treatment of intracavernous ICA aneurysms has evolved from simple ligation of the ICA in the neck3,18,37,38,40,41,44-46 to more refined procedures, including endo vascular (detachable balloon or coil embolization)23,60 or surgical therapies (direct clipping or parent-vessel clip reconstruction,8-11 aneurysmorrhaphy,9,11,50 or aneurysm trapping after bypass grafting).8,33,50,52,53,56
Historically, these lesions have been diagnosed on cerebral angiography; however, the shortcomings of this imaging modality in this population warrant discussion. In patients whose ophthalmic artery has an intracavernous origin, an extracavernous ICA aneurysm could be incorrectly localized as having an intracavernous origin. Cerebral angiography may underestimate the size of an aneurysm with a luminal thrombus. A partially thrombosed saccular aneurysm may be diagnosed incorrectly as a fusiform aneurysm due to its serpiginous lumen, while a completely thrombosed aneurysm may not be visualized angiographically. Both computed tomography (CT) and magnetic resonance (MR) imaging can be used to determine the true size of a partially or completely thrombosed aneurysm. MR imaging better demonstrates intradural extensions of aneurysms. CT scanning better visualizes subarachnoid and/or intracerebral hemorrhage in cases of acute rupture; furthermore, a CT scan bone algorithm shows bony erosion or remodeling in patients with large or giant aneurysms.
Treatment is considered ideal if it eliminates the aneurysm from the circulation while preserving normal flow through the parent vessel and its branches. These requirements are best accomplished by placing a clip directly across the aneurysm neck. Surgical treatment aimed at direct exposure and clipping of these aneurysms, however, represents a formidable challenge because of the intimate relationship of the ICA to the third through sixth cranial nerves within the cavernous sinus7,14,18,36,40,57,66 and because of the risk of bleeding from the cavernous sinus. The anatomical relationships of vital neurovascular structures in the cavernous sinus region are complex, and a detailed knowledge of the anatomy is essential for safe surgical approaches to this area.7,14,18,29,36,40,57,66
Triangles and Routes of Entry
A number of triangular spaces have been described as surgical corridors to enter the cavernous sinus safely. These triangles include the space between the optic nerve and the oculomotor nerve (anteromedial triangle),9,10 between the supraclinoid ICA and porus oculomotorius (medial or Hakuba’s triangle),20 between the oculomotor and the trochlear nerves (paramedian triangle),9,10 between the trochlear nerve and the first division of the trigeminal nerve (lateral or Parkinson’s triangle),42 between the first and second divisions of the trigeminal nerve (anterolateral or Mullan’s triangle),39 or between the second and the third divisions of the trigeminal nerve (lateral triangle).17 In order to expose the petrous portion of the carotid artery (C3 segment), the bony area bounded by the greater superficial petrosal nerve, the third division of the trigeminal nerve, and an arbitrary line between the foramen spinosum and the arcuate eminence of the petrous bone (posterolateral or Glasscock’s triangle) may be drilled off.19
Direct Surgery and Aneurysm Clipping
Direct surgical approaches to the lesions located within the cavernous sinus improved rapidly after Parkinson reported the initial approach in 1965.42 The technique required hypothermic circulatory arrest, which provided a bloodless surgical field that facilitated easy dissection of the ICA and the cranial nerves. The technique, however, carried the inherent risks associated with full heparinization, profound hypothermia, extracorporeal circulation, and cardiac arrest.5
To attack the cavernous sinus directly without the need for special measures such as hypothermic circulatory arrest, Dolenc9-11 used a combination of three different routes: the pterional, the subtemporal, and the petrosal approaches. The lesser sphenoid wing, orbital roof, anterior clinoid process, and roof of the optic canal are removed extradurally and the cavernous sinus is exposed. Alternatively, the falciform ligament may be incised intradurally, with subsequent drilling of the anterior clinoid. Arterial control is gained by drilling the petrous portion of the ICA out of its bony canal proximally (when a C5 to C3 bypass is planned) and by exposing the supraclinoid portion of the ICA in the subarachnoid space. The cranial nerves, including the oculomotor and trochlear nerves and the first division of the trigeminal nerve, are dissected from posterior to anterior and mobilized before the cavernous sinus is entered. This approach therefore lessens the risk of injuring these nerves. Other advantages of this approach include better proximal and distal control of the ICA within the same surgical field and a gradual and progressive control of cavernous sinus bleeding. The major disadvantage, however, is the need for significant brain retraction to expose the cavernous sinus and the petrous ICA. Other potential complications are the loss of lacrimal gland function and temporary or permanent facial nerve palsy due to injury or traction of the greater superficial petrosal nerve, respectively. Injury to the semicircular canals (with resultant hearing loss), the trigeminal nerve, or the ICA during drilling and dissection in the petrous bone is also possible.
In the present series, the excellent overall outcome of the five patients who underwent direct clipping compares favorably with the results from other surgical series.1,8,31 In certain cases, it may be possible to clip an aneurysm that at first inspection appears impossible to clip by using the following adjuncts: deep hypotension with hypothermic circulatory arrest,22,54 temporary proximal occlusion of the ICA,64 decompression of the aneurysm,4,15 or aneurysmorrhaphy.9,11,50 Deep hypotension without other planned intraoperative adjuncts is not ideal for most patients and should be avoided if preoperative neurological and cardiovascular functions are compromised. Although risky in some patients, hypothermic circulatory arrest may be a useful adjuvant to the treatment of certain difficult aneurysms in selected patients. We have found the temporary occlusion of the ICA, under controlled circumstances, to be helpful during aneurysm dissection and clip application.
ICA Trapping/Occlusion and Revascularization
Certain aneurysms may not be amenable to clipping for several reasons: their large size, the lack of a discrete neck, the presence of calcification or atheromatous plaques within the wall of the ICA or aneurysm itself, or other surgical or technical limitations. Alternative surgical procedures for treating such “unclippable” aneurysms include proximal vessel occlusion, trapping, or excision. Techniques that sacrifice the parent vessel are associated with the risk of consequent hemodynamic compromise and ischemic complications.18,37,41,44-46 Therefore, we believe that safe treatment of “unclippable” aneurysms with proximal vessel ligation or trapping requires revascularization to maintain blood flow to the involved territories and to protect against ischemic complications. Many reports2,12,13,21,25,30,55,58,59,61-63,67 have demonstrated good overall outcomes in patients undergoing trapping of their aneurysms with occlusion of the parent vessel and revascularization. Of the 16 patients in this series treated with some combination of these techniques, 14 (87.5%) had good overall outcomes.
Revascularization appears to be a reasonable option when surgical occlusion of the ICA is planned. Bypass surgical expertise, however, is a requirement in adopting this “universal” bypass approach to the sacrifice of parent ICAs. To identify patients who would or would not tolerate sacrifice of the parent ICA (selective revascularization), Sekhar and coworkers33,52 used the clinical balloon test occlusion (BTO) coupled with an ICA-occluded stable xenon/CT cerebral blood flow (CBF) study to assess cerebrovascular reserve before surgery. Based on early data, they developed a preoperative protocol to group patients according to low, moderate, or high risk of stroke.24,34 According to these authors, patients who tolerate BTO and show only mild symmetric decreases in hemispheric CBF (>30 ml/100 g/min) have a low risk and do not usually require revascularization. Patients who tolerate BTO but who have a marked asymmetric decrease in hemispheric CBF (<30 ml/100 g/min) have a moderate risk and typically require revascularization. Patients who develop neurological deficits during BTO are at high risk and require revascularization. In a later article,51 however, the same authors also reported ischemic complications in low-risk patients (BTO: Group I) who technically “passed” their BTO and underwent parent-vessel occlusion.
Based on our experience with revascularization in patients with intracranial aneurysms30 and on the unreliability of the BTO, we believe that revascularization is reasonable for all patients (i.e., universal revascularization) who have undergone occlusion of a major parent artery because the morbidity and mortality rates associated with revascularization are relatively low in experienced hands. The incidence of acute or delayed stroke associated with revascularization is likely lower than the cumulative risks of not revascularizing patients who tolerate BTO (i.e., the risk of ICA sacrifice = the risks of BTO + angiography + long-term ischemia + de novo aneurysm formation).30 In this series, all 16 patients who had occlusion of the ICA as a part of their treatment underwent prophylactic revascularization.
If an STA-MCA bypass was thought to provide inadequate flow after ICA occlusion, a petrous-to-supraclinoid ICA (C5-C3) bypass was performed using a saphenous-vein graft. Although technically demanding, this type of bypass is shorter, entirely intracranial, less vulnerable to injury, and more likely to remain patent than long saphenous-vein grafts. In this series, other types of bypasses were used when a normal, healthy petrous ICA was unavailable (two cases) or anastomosis was difficult (three cases), or to treat contralateral ICA occlusive disease in conjunction with trapping and a C5-C3 ICA bypass for an ipsilateral aneurysm.
Alternative Treatments
Patients with unclippable aneurysms, especially those who are not candidates for an occlusion/trapping and bypass procedure, may undergo other forms of therapy, including endovascular treatment23,60 and aneurysm wrapping.6,65 In the last few years, endovascular treatment has evolved rapidly and provides a viable option in the management of these lesions. Coil occlusion of an aneurysm may achieve optimal results in carefully selected patients who have small-necked aneurysms. Giant aneurysms with wide necks can be treated by either endovascular coil or detachable balloon techniques, but the risk of occluding the parent vessel is high. Combined endovascular and conventional surgical techniques may be an increasingly important strategy in the treatment of symptomatic intracavernous ICA aneurysms. Endovascular therapy for proximal occlusion/trapping of the ICA after revascularization (Case 17) is efficacious for some cavernous aneurysms.
Certain complex aneurysms with friable walls or indiscrete necks may give rise to important perforating branches or may involve the entire circumference of the artery and consequently may be extremely difficult to manage. In such cases clipping is unsafe, and endovascular occlusion cannot be accomplished without sacrificing the parent artery; wrapping or reinforcing the aneurysm may be a reasonable alternative.6 Occasionally, partial wrapping may be considered to reinforce a small intradural extension of the aneurysm that is not amenable to clipping or to reinforce small unclipped segments of an aneurysm to avoid kinking the parent vessel or compromising one of its branches.65
Conclusion
Most aneurysms of the intracavernous ICA do not require surgical treatment and can be managed conservatively by close observation. Aneurysms that produce clinical manifestations by rupture (e.g., SAH, carotid-cavernous fistula or epistaxis) or by progressive enlargement with or without cranial nerve deficits (e.g., headache, facial and orbital pain, ophthalmoplegia or visual loss) as well as asymptomatic aneurysms that project into the subarachnoid space should be considered for surgical management. Direct clipping carries substantial risks of injury to the cranial nerves and the ICA and hence is reserved for aneurysms with intradural extensions. Large and giant aneurysms confined to the cavernous sinus are best treated with trapping and vascular bypass procedures. In our experience, the petrous-to-supraclinoid ICA bypass using an interposition saphenous-vein graft has been an effective and useful option for revascularization. As a “last-ditch” life-saving measure, parent ICA occlusion without revascularization may only be considered for patients who require treatment (i.e., with symptomatic cavernous ICA aneurysms, SAH, carotid-cavernous fistula, or epistaxis) but who are unsuitable candidates for surgery or for selective endovascular aneurysm occlusion
References
- al-Rodhan NR, Piepgras DG, Sundt TM, Jr: Transitional cavernous aneurysms of the internal carotid artery [Review]. Neurosurgery 33:993-998, 1993
- Ausman JI, Diaz FG, Sadasivan B, et al: Giant intracranial aneurysm surgery: The role of microvascular reconstruction. Surg Neurol 34:8-15, 1990
- Barr HW, Blackwood W, Meadows SP: Intracavernous carotid aneurysms. A clinical-pathological report. Brain 94:607-622, 1971
- Batjer HH, Samson DS: Retrograde suction decompression of giant paraclinoidal aneurysms. Technical note. J Neurosurg 73:305-306, 1990
- Baumgartner WA, Silverberg GD, Ream AK, et al: Reappraisal of cardiopulmonary bypass with deep hypothermia and circulatory arrest for complex neurosurgical operations. Surgery 94:242-249, 1983
- Bederson JB, Zabramski JM, Spetzler RF: Treatment of fusiform intracranial aneurysms by circumferential wrapping with clip reinforcement: Technical note. J Neurosurg 77:478-480, 1992
- Diaz FG, Ausman JI, Pearce JE: Ischemic complications after combined internal carotid artery occlusion and extracranial-intracranial anastomosis. Neurosurgery 10:563-570, 1982
- Diaz FG, Ohaegbulam S, Dujovny M, et al: Surgical alternatives in the treatment of cavernous sinus aneurysms. J Neurosurg 71:846-853, 1989
- Dolenc V: Direct microsurgical repair of intracavernous vascular lesions. J Neurosurg 58:824-831, 1983
- Dolenc VV: A combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg 62:667-672, 1985
- Dolenc VV: Surgery of vascular lesions of the cavernous sinus [Review]. Clin Neurosurg 36:240-255, 1990
- Drake CG: Giant intracranial aneurysms: Experience with surgical treatment in 174 patients. Clin Neurosurg 26:12-95, 1979
- Drake CG, Peerless SJ, Ferguson GG: Hunterian proximal arterial occlusion for giant aneurysms of the carotid circulation. J Neurosurg 81:656-665, 1994
- Ferguson GG, Drake CG: Carotid-ophthalmic aneurysms: The surgical management of those cases presenting with compression of the optic nerves and chiasm alone. Clin Neurosurg 27:263-307, 1980
- Flamm ES: Suction decompression of aneurysms. Technical note. J Neurosurg 54:275-276, 1981
- Fox AJ, Vinuela F, Pelz DM, et al: Use of detachable balloon for proximal artery occlusionin the treatment of unclippable cerebral aneurysms. J Neurosurg 66:40-46, 1987
- Fukushima T, Day JD: Surgical management of tumors involving the cavernous sinus, in SchmidekHH, Sweet WH (eds): Operative Neurosurgical Techniques. Philadelphia: W.B. Saunders, 1995, pp 493-510
- Giannotta SL, McGillicuddy JE, Kindt GW: Gradual carotid artery occlusion in the treatment of inaccessible internal carotid artery aneurysms. Neurosurgery 5:417-421, 1979
- Glasscock ME, III: Exposure of the intra-petrous portion of the carotid artery, in HambergerCA, Wersall J (eds): Disorders of the Skull Base Region. Proceedings of the Tenth Nobel Symposium. Stockholm:Almqvist & Wiksell, 1969, pp 135-143
- Hakuba A, Matsuoka Y, Suzuki T, et al: Direct approaches to vascular lesions in the cavernous sinus via the medial triangle, in Dolenc VV (ed): The Cavernous Sinus. A Multidisciplinary Approach to Vascular and Tumorous Lesions. New York: Springer-Verlag, 1987, pp 272-284
- Heros RC: Management of giant paraclinoid aneurysms, in Kikuchi H, Fukushima T, Wata nabeK (eds): Intracranial Aneurysms. Japan: Nishimura, 1986, pp 273-282
- Heros RC: Surgical management of unclippable intracranial aneurysms, in Schmidek HH, SweetWH (eds): Operative Neurosurgical Techniques. Philadelphia: W.B.Saunders, 1995, pp 1113-1125
- Higashida RT, Halbach VV, Dowd C, et al: Endovascular detachable balloon embolization therapy of cavernous carotid artery aneurysms: Results in 87 cases. J Neurosurg 72:857-863, 1990
- Horton JA, Jungreis CA, Pistoia F: Balloon test occlusion, in Sekhar LN, Janecka IP (eds):Surgery of Cranial Base Tumors. New York: Raven, 1993, pp 33-36
- Hosobuchi Y: Giant intracranial aneurysms, in Wilkins RH, Rengachary SS (eds): Neurosurgery.New York: McGraw-Hill, 1985, pp 1404-1414
- Hunt WE, Hess RM: Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg 28:14-20, 1968
- Jane JA, Winn HR, Richardson AE: The natural history of intracranial aneurysms: Rebleeding rates during the acute and long term period and implication for surgical management. Clin Neurosurg 24:176-184,1977
- Jennett B, Bond M: Assessment of outcome after severe brain damage. Lancet 1:480-484,1975
- Kiris T, Sankhla SK, Lawton MT, et al: Microsurgical anatomy of the cavernous sinus. BNIQuarterly 12:4-14, 1996
- Lawton MT, Hamilton MG, Morcos JJ, et al: Revascularization and aneurysm surgery: Current techniques, indications, and outcome. Neurosurgery 38:83-94, 1996
- Linskey ME, Sekhar LN, Hirsch W, Jr., et al: Aneurysms of the intracavernous carotid artery:Clinical presentation, radiographic features, and pathogenesis. Neurosurgery 26:71-79, 1990
- Linskey ME, Sekhar LN, Hirsch WL, Jr., et al: Aneurysms of the intracavernous carotid artery:Natural history and indications for treatment. Neurosurgery 26:933-938, 1990
- Linskey ME, Sekhar LN, Horton JA, et al: Aneurysms of the intracavernous carotid artery:A multidisciplinary approach to treatment.J Neurosurg 75:525-534, 1991
- Linskey ME, Sekhar LN, Sen C: Cerebral revascularization in cranial base surgery, in SekharLN, Janecka IP (eds): Surgery of Cranial Base Tumors. New York: Raven, 1993, pp 45-68
- Locksley HB: Natural history of subarachnoid hemorrhage, intracranial aneurysms, and arteriovenous malformations. J Neurosurg 25:321-368, 1966
- Matsuda M, Shiino A, Handa J: Rupture of previously unruptured giant carotid aneurysm after superficial temporal-middle cerebral artery bypass and internal carotid occlusion. Neurosurgery 16:177-184,1985
- Miller JD, Jawad K, Jennett B: Safety of carotid ligation and its role in the management of intracranial aneurysms. J Neurol Neurosurg Psychiatry 40:64-72, 1977
- Morley TP, Barr HW: Giant intracranial aneurysms: Diagnosis, course, and management. ClinNeurosurg 16:73-94, 1969
- Mullan S: Treatment of carotid-cavernous fistulas by cavernous sinus occlusion. J Neurosurg50:131-144, 1979
- Nishioka H: Results of the treatment of intracranial aneurysms by occlusion of the carotidartery in the neck. J Neurosurg 25:660-704, 1966
- Odom GL, Tindall GT: Carotid ligation in the treatment of certain intracranial aneurysms.Clin Neurosurg 15:101-116, 1968
- Parkinson D: A surgical approach to the cavernous portion of the carotid artery. Anatomical studies and case report. J Neurosurg 23:474-483, 1965
- Parkinson D: Surgical approach to cavernous sinus aneurysms, in Pia HW, Langmaid C, ZierskiJ (eds): Cerebral Aneurysms. Advances in Diagnosis and Therapy. Berlin: Springer-Verlag, 1979, pp 224-228
- Pozzati E, Fagioli L, Servadei F, et al: Effect of common carotid ligation on giant aneurysmsof the internal carotid artery. Computerized tomography study. J Neurosurg 55:527-531, 1981
- Pritz MB: Cardiopulmonary monitoring during graded cervical internal carotid artery occlusion: Physiological results and therapeutic implications. Neurosurgery 8:520-524, 1981
- Roski RA, Spetzler RF, Nulsen FE: Late complications of carotid ligation in the treatment of intracranial aneurysms. J Neurosurg 54:583-587, 1981
- Sahs AL, Perret GE, Locksley HB, et al: Intracranial Aneurysms and Subarachnoid Hemorrhage: A Cooperative Study. Philadelphia: J.B. Lippincott: 1969
- Sankhla SK, Vishteh AG, Lawton MT, et al: Surgical management of intracavernous carotid artery aneurysms. Advances in Clinical Neurosciences 6: 417-440, 1996
- Scialfa G, Vaghi A, Valsecchi F, et al: Neuroradio logical treatment of carotid and vertebral fistulas and intracavernous aneurysms. Technical problems and results. Neuroradiology 24:13-25, 1982
- Sekhar LN, Linskey ME, Sen CN, et al: Surgical management of lesions within the cavernoussinus. Clin Neurosurg 37:440-489, 1991
- Sekhar LN, Patel SJ: Permanent occlusion of the internal carotid artery during skull-base and vascular surgery: Is it really safe?Am J Otol 14:421-422, 1993
- Sekhar LN, Sen CN, Jho HD: Saphenous vein graft bypass of the cavernous internal carotid artery. J Neurosurg 72:35-41, 1990
- Sen CN, Sekhar LN: Direct vein graft reconstruction of the cavernous, petrous and upper cervical internal carotid artery: Lessons learned from 30 cases. Neurosurgery 30:732-743, 1992
- Solomon RA, Smith CR, Raps EC, et al: Deep hypothermic circulatory arrest for the management of complex anterior and posterior circulation aneurysms. Neurosurgery 29:732-737, 1991
- Spetzler RF, Carter LP: Revascularization and aneurysm surgery: Current status. Neurosurgery16:111-116, 1985
- Spetzler RF, Fukushima T, Martin N, et al: Petrous carotid-to-intradural carotid saphenousvein graft for intracavernous giant aneurysm, tumor, and occlusive cerebrovascular disease. J Neurosurg 73:496-501,1990
- Spetzler RF, Roski RA, Schuster H, et al: The role of EC-IC in the treatment of giant intracranial aneurysms. Neurol Res 2:345-359, 1980
- Spetzler RF, Schuster H, Roski RA: Elective extracranial-intracranial arterial bypass in the treatment of inoperable giant aneurysms of the internal carotid artery. J Neurosurg 53:22-27, 1980
- Spetzler RF, Selman W, Carter LP: Elective EC-IC bypass for unclippable intracranial aneurysms.Neurol Res 6:64-68, 1984
- Standard SC, Guterman LR, Chavis TD, et al: Endovascular management of giant intracranial aneurysms. Clin Neurosurg 42:267-293, 1994
- Sundt TM, Jr., Piepgras DG: Surgical approach to giant intracranial aneurysms: Operative experience with 80 cases. J Neurosurg 51:731-742, 1979
- Sundt TM, Jr., Piepgras DG: Surgical management of giant intracranial aneurysms, in KikuchiM, Fukushima T, Watanabe K (eds):Intracranial Aneurysms. Japan: Nishimura Co. 1986, pp 324-335
- Sundt TM, Jr., Piepgras DG, Marsh WR, et al: Saphenous vein bypass grafts for giant aneurysms and intracranial occlusive disease. J Neurosurg 65:439-450, 1986
- Suzuki J, Kwak R, Okudairo Y: The safe time limit of temporary clamping of cerebral arteries in the direct surgical treatment of intracranial aneurysms, in Suzuki J (ed): Cerebral Aneurysms. Tokyo: Neuron Publishing Co. 1979, pp 325-330
- Todd NV, Tocher JR, Jones PA, et al: Outcome following aneurysm wrapping: A 10-year follow-up review of clipped and wrapped aneurysms. J Neurosurg 70:841-846, 1989
- Umansky F, Nathan H: The lateral wall of the cavernous sinus. With special reference to the nerves related to it. J Neurosurg 56:228-234, 1982
- Yasargil MG: Giant intracranial aneurysms, in Yasargil MG (ed): Clinical Considerations, Surgery of the Intracranial Aneurysms and Results. Stuttgart: Georg Thieme Verlag, 1984, pp 296-304.
