
The Laparoscopic Approach for Instrumentation and Fusion of the Lumbar Spine
Authors
Curtis A. Dickman, MD
Volker K. H. Sonntag, MD
James C. Russell, MD†
Division of Neurological Surgery, Barrow Neurological Institute
†Department of General Surgery, Mercy Healthcare Arizona, Phoenix, Arizona
Abstract
Laparoscopy can be used to effectively internally fixate and fuse the L4-L5 and L5-S1 spinal levels, using interbody threaded fusion devices. The techniques are relatively straightforward; however, they are associated with a definite learning curve. Transperitoneal endoscopy is an excellent alternative to open laparotomy and posterior approaches for instrumentation and fusion of the lower lumbar spine. This technique preserves the normal muscle tissue of the abdominal wall, reduces approach-related morbidity of spine surgery, and avoids devascularizing paraspinal muscle and stripping muscle from the spinal surface. These features reduce the amount of postoperative pain, shorten the recovery, facilitate earlier return to activity, and improve cosmetic results compared to open approaches. The laparoscopic approach represents a significant advance in the surgical armamentarian for fixating and fusing the lumbosacral spine.
Key Words : BAK device, endoscopy, laparoscopy, lumbar spinal instability, minimally invasive surgery, spinal fusion, spine surgery, threaded interbody fusion cages
Clinically, laparoscopy became popular in the late 1980s and has been used in general surgery and obstetrics for a variety of indications including cholecystectomy, appendectomy, hernia repair, bowel resection, tubal ligation, and hysterectomy among others. Laparoscopy was developed as an alternative to open laparotomy to reduce the morbidity to approach the peritoneal and pelvic contents. Rather than transecting the abdominal muscles and destroying normal healthy tissue of the abdominal wall, narrow portals are inserted to access the peritoneal cavity. The portals separate and preserve the muscles. Consequently, postoperative pain and recovery time are reduced, and patients have smaller scars compared to laparotomy. This article reviews the laparoscopic approach for anterior interbody fixation and fusion of the lumbar and lumbosacral spine, focusing on operative techniques.
Indications and Contraindications
Patients are selected based on indications similar to those used for open techniques for lumbar interbody fusion devices (see Internal Fixation and Fusion of the Lumbar Spine Using Threaded Interbody Cages in this issue). The surgical indications for laparoscopic interbody fusion include pathology at the levels of L4-L5 or L5-S1, including nonunion (pseudarthrosis) of a posterior lumbar or lumbosacral fusion; Grade I or II spondylolisthesis (degenerative or congenital spondylolisthesis); and disc degeneration associated with neural foraminal stenosis that causes radicular pain, incapacitating mechanical back pain, or both. These problems may be considered for surgical treatment if extensive nonoperative treatment with physical therapy, bracing, anti-inflammatory medications, weight loss, lumbar strengthening exercises, modification of activity, and other modalities has failed.
The level of a patient’s pathology also plays a role in the decision to pursue a laparoscopic approach. The easiest level to access pathology through the laparoscopic approach is L5-S1 because the aorta and vena cava usually bifurcate into the common iliac vessels rostral to the L5-S1 disc space. The L4-L5 disc level also can be exposed laparoscopically, but it is more difficult because more extensive vascular mobilization tends to be required. Occasionally, a high bifurcation of the vessels simplifies the approach to L4-L5. The position of the vessels often can be judged on preoperative computed tomography scans or magnetic resonance images of the spine and abdomen. Lumbar disc levels above L4-L5 are most easily approached anteriorly with methods other than laparoscopy (i.e., posterior or retroperitoneal approaches) because the viscera and vascular structures prohibit laparoscopic access.4,9
Contraindications to laparoscopy include morbid obesity, a prior laparotomy, extensive peritoneal adhesions, active neoplasms or infections of the peritoneal cavity, or medical illnesses that would prohibit surgery.
Surgical Technique
Preoperative Preparation
The evening before surgery, the patients are treated with cathartic agents (e.g., magnesium citrate) to evacuate the large bowel. Emptying the colon and small intestines facilitates their intraoperative mobilization to expose the spine. Arterial and central venous catheters, a Foley catheter, and a nasogastric tube are placed. Pneumatic antiembolism compression stockings are applied to the legs to avoid venous stasis.
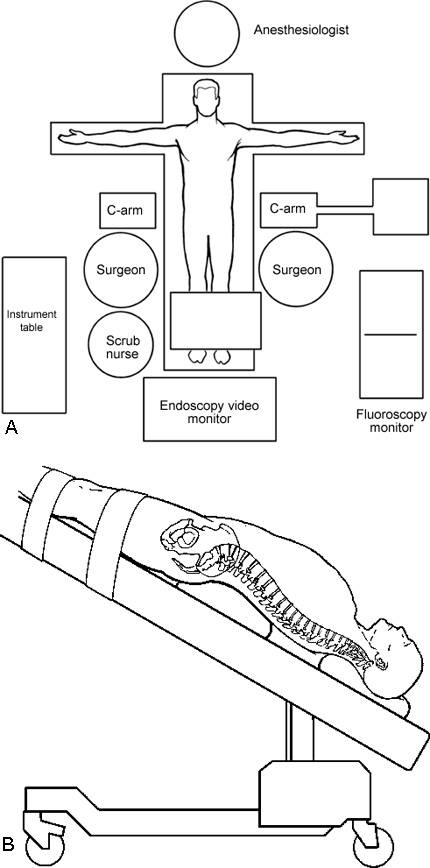
The patient is anesthetized and placed in a supine position on a radiolucent operating table (Fig. 1). A roll is placed under the lumbar spine to accentuate the lumbar lordosis. The feet are anchored to the operating table using kerlex gauze because the table is tilted into a steep Trendelenburg position. During the procedure, gravity then helps to retract the intestines rostrally out of the pelvis and lower abdomen.
The abdomen, suprapubic region, and iliac crests are sterilely scrubbed using a routine preparation. To facilitate the procedure and to reduce the amount of operative time, the iliac bone grafts are harvested before the laparoscopy is performed. The anterior iliac crest usually provides enough graft material for single-level fusions (i.e., two cages). For two-level fusions, the posterior iliac crest is needed.
Portal Placement
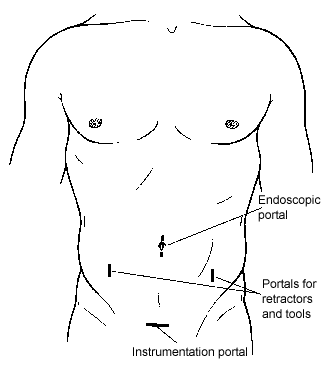
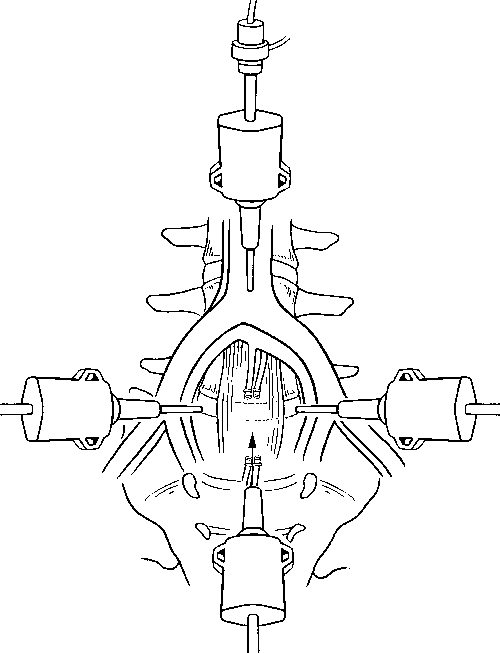
The peritoneal cavity can be exposed using CO2 insufflation or gasless laparoscopic methods. The former is used most often. Four small portal incisions are used to expose and visualize the surgical field and to insert tools. One 10-mm incision, one 18- to 20-mm incision, and two 5-mm incisions are made in the abdomen for insertion of the portals (Fig. 2).
A 1- to 2-mm incision is made at the umbilicus, and a Veress insufflating needle is inserted into the abdomen. The peritoneal cavity is insufflated with CO2 to a pressure of 10 to 15 mm Hg. A 10-mm portal for the endoscope is inserted into the umbilical incision. A 10-mm diameter, rigid, high-resolution endoscope is inserted and the contents of the peritoneal cavity are inspected.
The other three portals are inserted under direct visualization with the endoscope. A 30°-angled endoscope is more useful than a 0° telescope for visualizing this procedure. Two 5-mm portals for tools and retractors are inserted in the lateral abdominal wall, caudal to the umbilicus. The portals are positioned lateral to the rectus sheath 1 to 2 cm rostral to the disc level(s) to be accessed. Fluoroscopy is used to help position the midline 18- to 20-mm suprapubic incision that is used to insert the spinal instrumentation.
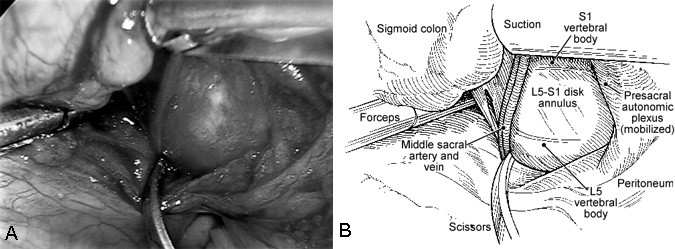
The lumbar spine is exposed by incising the posterior peritoneum at the base of the sigmoid mesocolon sagittally with endoscopic scissors (endoshears). The middle sacral artery and vein are identified over the L5-S1 disc space. The presacral plexus of autonomic nerve fibers is mobilized and preserved. Blunt dissection with an endoscopic cotton-tipped dissector (i.e., cherry or peanut dissector) is used to sweep the nerves laterally from the surface of the disc space (Fig. 4).
Cauterization is rarely needed for hemostasis with this approach, and it should be avoided whenever possible near the disc space. Monopolar cauterization should never be used. Only bipolar cauterization should be used near the disc space and presacral autonomic plexus. By doing so, the rare complication of retrograde ejaculation in males (less than 2%) can be minimized.
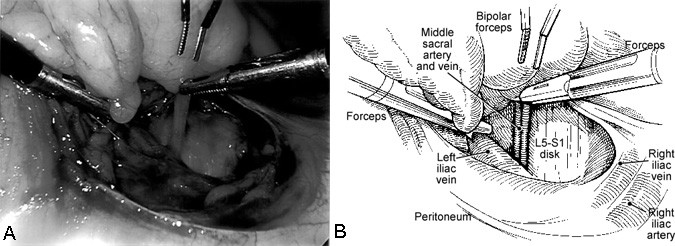
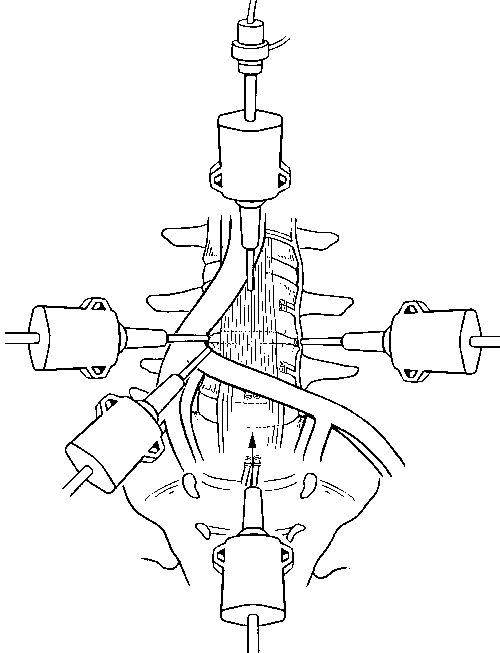
The L5-S1 disc space is exposed rather easily. The middle sacral artery and vein are mobilized, ligated with endoscopic hemoclips or endoscopic suture ligatures, and transected (Fig. 5). The medial surfaces of the iliac arteries and veins also may need to be retracted gently using endoscopic vascular retractors to fully expose the L5-S1 disc space (Fig. 6).
The L4-L5 disc space requires more expertise to expose than L5-S1. The sigmoid colon is mobilized, and the mesentery of the sigmoid colon is incised 3 to 4 cm rostral to the incision for the L5-S1 disc space on the left side of the aorta and the left common iliac artery and vein. The ascending lumbar vessels and segmental vessels must be ligated and transected to mobilize and retract the iliac vessels, aorta, and vena cava to the right side to expose the L4-L5 disc fully (Fig. 7).
Insertion of Hardware
The exposure of the disc space and mobilization of the blood vessels usually are the longest and the technically most difficult portions of the laparoscopic operation. In contrast, inserting the hardware is relatively easy. The techniques to insert the threaded interbody fusion cages closely resemble the techniques used during open laparotomy.
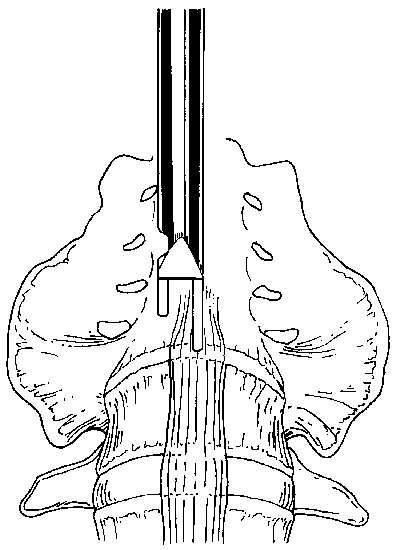
After the disc space is exposed, the suprapubic skin incision is made in a position that will allow the hardware to be placed exactly parallel to the vertebral end plates of the disc space. The positions of the skin incision, hardware, and tools are verified using a C-arm fluoroscopy unit. A Steinmann pin is inserted in the midline through the suprapubic incision to assess the trajectory of the tools and to identify the midline of the disc space (using anteroposterior (AP) and lateral fluoroscopy). Insertion of the Steinmann pin and all other surgical maneuvers are observed directly with the endoscope and with fluoroscopy.
The suprapubic incision is lengthened to approximately 20 mm to fit the operating trochar for the hardware. The operating trochar is inserted over an introducing trochar. It is wide enough to fit the tools and cages.

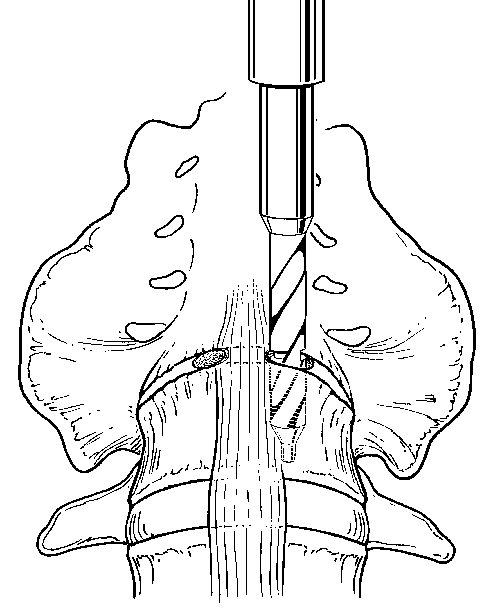
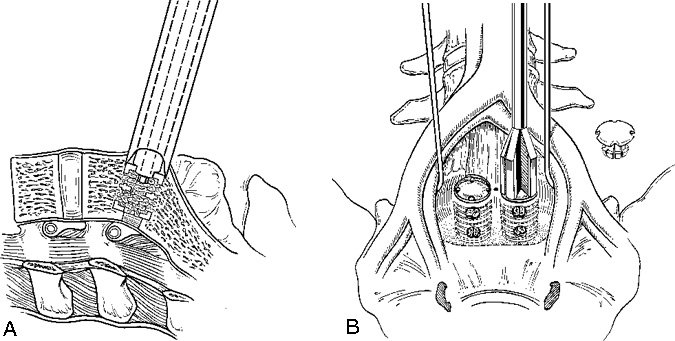
The iliac vessels are gently retracted laterally with endoscopic vein retractors while the disc space is prepared and the hardware is inserted. The annulus of the disc space is marked using an alignment guide to identify the right and left entrance sites for the two threaded cages (Fig. 8). Holes are drilled at each entry site using an 8-mm diameter drill. The drill holes are oriented identical to the intended trajectory for the cages (Fig. 9). Disc material is removed from the drill holes with disc rongeurs (Fig. 10).
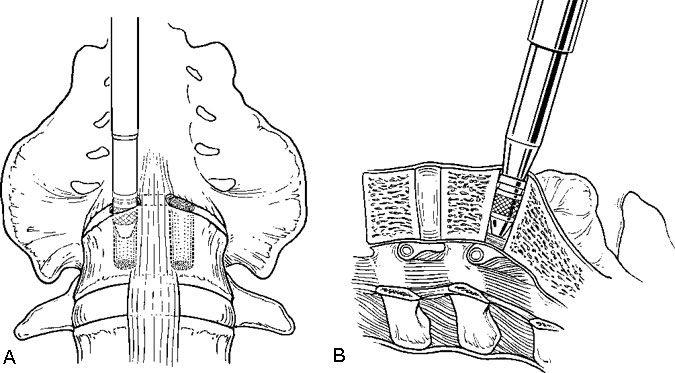
Distraction plugs are inserted sequentially into the holes in the disc spaces to reduce any spondylolisthesis, to distract the neural foramen, to apply tension to the annulus and ligaments, and to create space to fit the interbody fusion cages (Fig. 11). The distraction plugs should fit tightly into the disc space and should be difficult to remove. The diameter of the threaded interbody implant should be at least 3 mm wider than the diameter of the distraction plug so that it fits tightly.
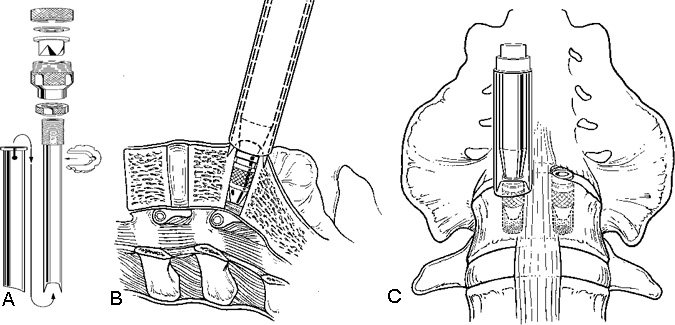
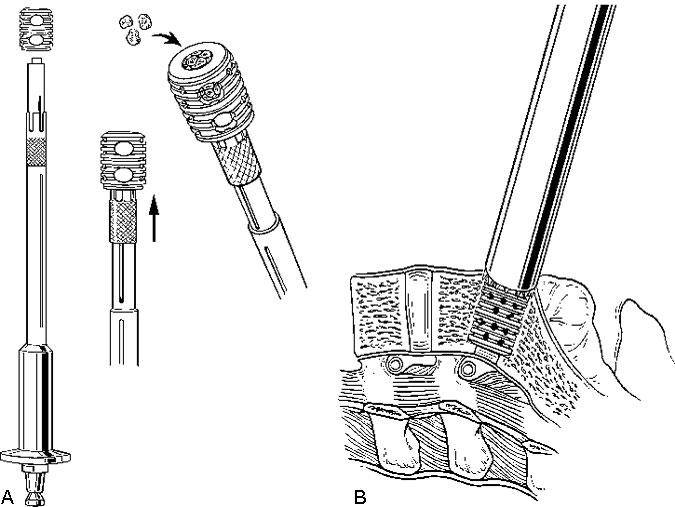
After the disc space has been distracted, a drill tube trochar is inserted. The trochar has a valve mechanism to preserve the pressurized CO2 insufflation. The drill tube has a protective outer sheath. The inner sheath has retractable teeth to anchor the drill tube to the disc space (Fig. 12). After the drill tube guide is inserted, one distraction plug is removed and the pilot hole for the cage is reamed with a drill (Fig. 13). The pilot hole is tapped to cut the thread profile into the bone (Fig. 14).
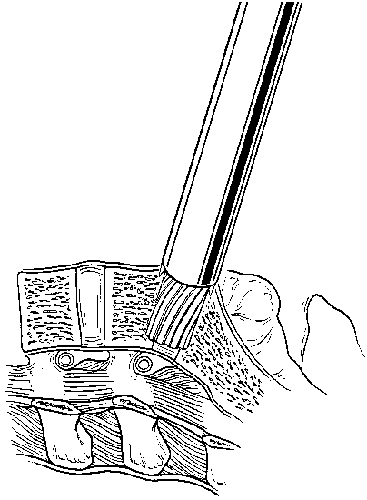
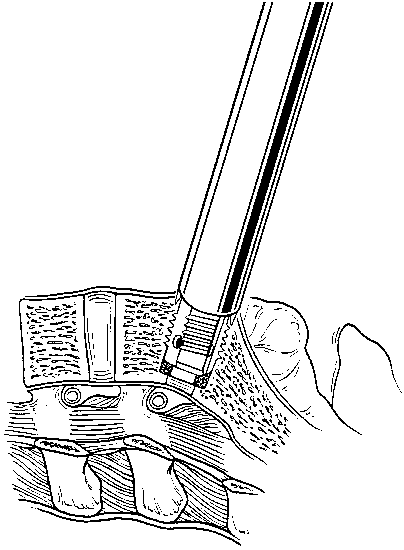
The cage is packed with cancellous bone graft and inserted into the pilot hole (Fig. 15). This procedure is repeated on the contralateral side after the second distraction plug has been removed. After both cages have been positioned, the anterior chambers of the implants are packed full of bone graft (Fig. 16). The anterior surfaces of the cages are covered with bone graft so that bone bridging can be assessed postoperatively. All maneuvers for preparing and inserting the cages are monitored using AP and lateral fluoroscopic imaging.
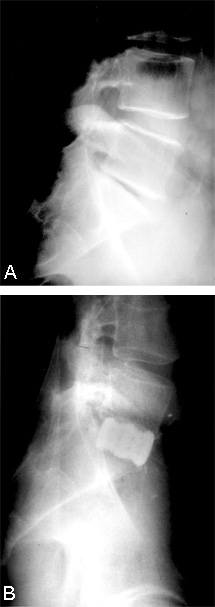
After the implants and grafts are inserted, their final position is assessed endoscopically and radiographically (Figs. 17 and 18). The implants are recessed from the anterior and posterior margins of the disc space. The surgical site is inspected and hemostasis is obtained.
Closure
The posterior peritoneum is closed using endoscopic suturing techniques or endoscopic clips. The CO2 insufflation is reduced and the contents of the peritoneal cavity are inspected to assure that hemostasis is obtained. Venous bleeding can be masked by CO2insufflation; therefore, the veins must be inspected carefully after the insufflation pressure is reduced.
The portals are removed and the peritoneum of all portal incisions that are 10 mm diameter or wider is closed endoscopically to prevent an incisional hernia. The incisions are closed with subcutaneous and subcuticular sutures. The fascia of the 20-mm suprapubic incision is also closed with interrupted absorbable sutures.
Postoperative Care
After the patients’ stability has been assessed in the recovery room, they are transferred to a general care ward. An intensive care unit (ICU) is seldom required. During the first 24 hours, pain is managed with a patient-controlled analgesic pump. Patients can be mobilized with a lumbar orthosis on the first postoperative day.
Discussion
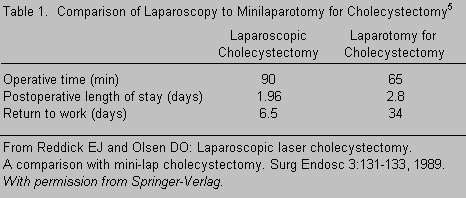
Laparoscopy is an established, well-accepted, clinically useful surgical technique to access and dissect structures within the peritoneal compartment. It already has widespread clinical applications in general surgery and obstetrics. Its clinical merits of smaller incisions, no muscle transection, reduced postoperative pain, and faster recoveries also apply when the technique is used for spinal access. The approach-related morbidity is less than the morbidity associated with laparotomy or with posterior approaches to access the lumbosacral spine. The most notable example of the benefits of laparoscopy is the markedly reduced recovery time after laparoscopic cholecystectomy (Table 1).5,10

Laparoscopic surgery to fuse the lumbar and lumbosacral spine is a new treatment option that appears to have significant clinical benefits. The technique requires special training and the participation of a general surgeon who is skilled in laparoscopy to access the spine and to mobilize the viscera and blood vessels. Teaming a laparoendoscopic access surgeon and a spinal surgeon together combines skills that optimize the benefits and minimize the complications for patients.
Clinical Outcomes
Threaded interbody fusion devices have been approved by the Federal Drug Administration (FDA) for the applications of anterior lumbar interbody fusion (ALIF), posterior lumbar interbody fusion (PLIF), and laparoscopic ALIF. The devices are safe and effectively stabilize and fuse the lumbosacral spine.
Under an FDA-approved Investigational Device Exemption (IDE) study protocol,7 the results of laparoscopic application of threaded interbody fusion cages have been compared to open application of these devices. The results of 80 laparoscopic fusions were compared to a large group of patients undergoing open anterior or posterior fusions with threaded fusion cages. The diagnostic indications for surgery were uniform across the groups and included prolonged (longer than 6 months) severe radicular and/or mechanical symptoms from L4-L5 or L5-S1 disc degeneration. Patients with spondylolisthesis were excluded.
The operative time for laparoscopic lumbar fusion was longer than the duration for open techniques by only 30 to 60 minutes. Blood loss, however, was significantly less with laparoscopy compared to the open techniques. There were no significant differences in the length of hospitalization.
There were few operative complications. Nine cases were converted to open laparotomy, primarily early in the surgeons’ operative experience. Three of these conversions were to control lacerations of the iliac vein. As the surgeons gained operative experience with these techniques, this complication was avoided. In two patients, the cage or a herniated disc impinged upon a nerve root and both required a second operation.
On July 22, 1997, the laparoscopic application of the BAK device for ALIF was approved by the FDA. Overall, the fusion rates, clinical outcomes, and complication rates associated with inserting the fixation cages have not been significantly different from those associated with open applications.1-3,6-8,12,13 For a more detailed discussion, see Internal Fixation and Fusion of the Lumbar Spine Using Threaded Interbody Cages in this issue.
Laparoscopic interbody fusion cages have also been compared to posterolateral fusion with pedicle screws.11 In a prospective randomized study, patients treated with laparoscopic anterior interbody fusion cages had significantly improved clinical results compared to patients treated with pedicle screws. Their hospital stays were shorter, they experienced less pain, and they returned to work more quickly than their counterparts with pedicle screws.
Recommendations
The laparoscopic technique has a definite learning curve. The procedures, however, can be mastered with dedicated training. Surgeons must be thoroughly familiar with the anatomy of the peritoneal cavity and its contents and the spinal and paraspinal anatomy. Advanced skills in endoscopic access and endoscopic dissection techniques are necessary. Both spine and access surgeons should attend didactic lectures and participate in surgical skill laboratories to learn the techniques of laparoscopic spine exposure and spine surgery. The general surgeon should be credentialed and skilled in laparoscopic surgery. These criteria must be met to ensure patient safety and successful clinical outcomes.
Conclusions
Laparoscopic spinal fixation and fusion represent a major advance in the treatment of lumbar spinal instability and degenerative disc disease. These procedures are performed using simple, straightforward surgical techniques, and they are biomechanically and clinically sound. Compared to open surgical procedures, they reduce approach-related morbidity and thereby facilitate a patient’s recovery and return to normal activity.
References
- Mathews HH, Evans MT, Kyles MK, et al: Laparoscopic discectomy with fusion. 9th Annual Meeting, North Am Spine Society, Minneapolis, MN, October 19-22, 1994, p. 63
- McAfee PC, Regan JR, Zdeblick T, et al: The incidence of complications in endoscopic anterior thoracolumbar spinal reconstructive surgery. A prospective multicenter study comprising the first 100 consecutive cases. Spine 20:1624-1632, 1995
- Novotny SR, Guyer RD, Regan JJ, et al: Laparoscopic-assisted anterior lumbar interbody fusion. 9th Annual Meeting, North Am Spine Society, Minneapolis, MN, October 19-22, 1994, p. 61
- Onimus M, Papin P, Gangloff S: Extraperitoneal approach to the lumbar spine with video assistance. Spine 21:2491-2494, 1996
- Reddick EJ, Olsen DO: Laparoscopic laser cholecystectomy. A comparison with mini-lap cholecystectomy. Surg Endosc 3:131-133, 1989
- Regan JJ, Ohnmeiss DD, Thompson T, et al: Laparoscopic-assisted fusion of the lumbar spine, a comparison to open procedures. 10th Annual Meeting, North Am Spine Society, Washington, DC, October 18-21, 1995, p. 169
- Regan JJ, Zdeblick T, Zucherman J, et al: Comparison of open versus laparoscopic fusion of the lumbar spine using the BAKTM threaded fusion cage. 10th Annual Meeting, North Am Spine Society, Washington, DC, October 18-21, 1995, p. 131
- Sachs BL, Schwaitzberg S: Laparoscopic lumbosacral discectomy and interbody fusion technique: Early clinical experience. 9th Annual Meeting, North Am Spine Society, Minneapolis, MN, October 19-22, 1994, p. 67
- Southerland SR, Remedios AM, McKerrell JG, et al: Laparoscopic approaches to the lumbar vertebrae. An anatomic study using a porcine model. Spine 20:1620-1623, 1995
- The Southern Surgeons Club: A prospective analysis of 1518 laparoscopic cholecystectomies. N Engl J Med 324:1073-1078, 1991
- Zdeblick TA, Ulschmid S, Dick JC: The surgical treatment of L5-S1 degenerative disc disease. A prospective randomized study. 10th Annual Meeting, North Am Spine Society, Washington, DC, October 18-21, 1995, p. 168
- Zucherman JF, Hsu K, Implacito D: Instrumented transperitoneal laparoscopic fusion, in Margulies JY, et al. (eds): Lumbosacral and Spinopelvic Fixation. Philadelphia: Lippincott-Raven, 1996, pp 579-585
- Zucherman JF, Zdeblick TA, Bailey SA, et al: Instrumented laparoscopic spinal fusion. Preliminary results. Spine 20:2029-2035, 1995
