
Depression in Parkinson’s Disease
Author
A. N. Lieberman, MD
Division of Neurology, Barrow Neurological Institute, Mercy Healthcare Arizona, Phoenix, Arizona
Abstract
Depression occurs in approximately 40% of Parkinson’s disease (PD) patients. It is an intrinsic component of PD unrelated to the degree of physical disability, and in 20% of depressed patients it precedes the onset of motor symptoms. In addition to an endogenous depression, PD patients may suffer from an exogenous depression related to job loss, retirement, or knowledge of a relative or friend with advanced PD. In many patients, PD is complicated by autonomic nervous system dysfunction, a sleep disorder, or a cognitive disorder. In the presence of these complications, it may be difficult to diagnose depression.
Key Words : anxiety, dementia, depression, movement disorder, Parkinson’s disease
Parkinson’s disease (PD) is characterized by the presence of two or more of four cardinal or primary symptoms: bradykinesia, rigidity, tremor, and postural instability.15 The cause of PD is unknown, but symptoms arise as dopaminergic neurons in the substantia nigra, noradrenergic neurons in the locus ceruleus, and cholinergic neurons in the nucleus basalis of Meynert degenerate.4 The neuronal degeneration is accompanied by Lewy bodies—intracytoplasmic inclusions that contain ubiquitin. An unequivocal and sustained response to levodopa usually distinguishes PD from several Parkinson-like disorders (Parkinson plus syndromes): multisystem atrophy (the Shy-Drager Syndrome), progressive supranuclear palsy, and corticobasilar degeneration. PD patients may have other symptoms such as hypomimia (facial masking), hypophonia (a low voice), and a stooped posture. These symptoms are secondary and represent the effects of bradykinesia or rigidity on specific sets of muscles. Depression is also common among Parkinson’s disease patients. Approximately 40% of PD patients experience depression accompanied by feelings of guilt, remorse, hopelessness, pessimism, and gloom. These feelings are independent of the patient’s age, the duration or severity of the disease, or the presence of cognitive impairment.
Symptoms common to both PD and depression make it difficult to determine if patients have one disorder or both. Thus, PD patients with hypomimia, hypophonia, and a stooped posture may appear depressed when they are not, while depressed patients with psychomotor retardation may appear to have PD when they do not. Finally, some PD patients appear depressed because their ability to recognize and express emotional cues is impaired, a condition known as aprosodia.12,26 A structured interview with patient, spouse, and caregiver and the use of depression rating scales (e.g., Beck, Hamilton) can help distinguish depressed from nondepressed PD patients. However, until a universal and specific test for depression is developed, one that distinguishes moderate or marked depression from transient disappointment and loneliness, there will be disagreement about the diagnosis of depression in PD. This article reviews features of depression in PD patients and therapeutic options for its treatment.
Prevalence of Depression
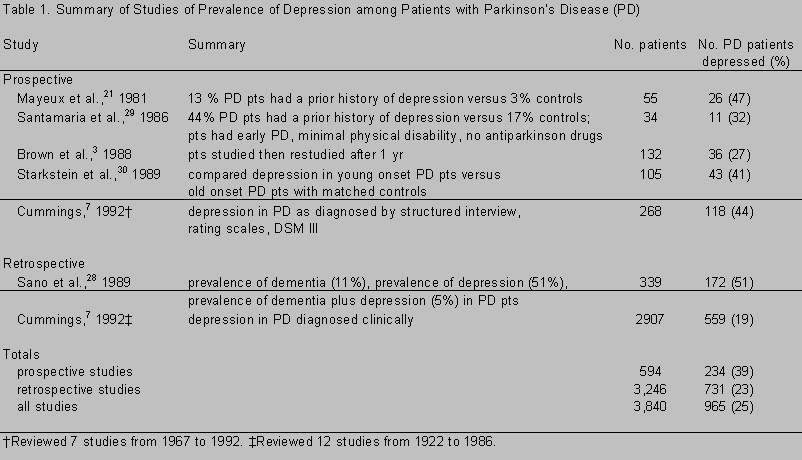
Depression is a primary, endogenous component of PD, not a reaction to a chronic and debilitating illness. In a review of 24 studies that encompassed 3,840 PD patients, 965 (25%) were moderately to markedly depressed (Table 1). 3,7,21,28-30 In 11 prospective studies, (15% of the 3,840 patients) in which PD patients were matched for age, gender, and physical disability, 39% were depressed. In 13 retrospective studies, 23% were depressed. In the prospective studies PD patients were matched with suitable controls, and depression, as defined by DSM-III criteria, was assessed by a structured interview or a validated depression rating scale. In these retrospective studies, PD patients were not always matched with controls. Furthermore, depression was not defined objectively, and it was only assessed clinically. The disparity in the findings between the two types of studies suggests that depression, when sought, may be more pervasive than imagined. A caveat, however, is that investigators who seek depression are more likely to find it.
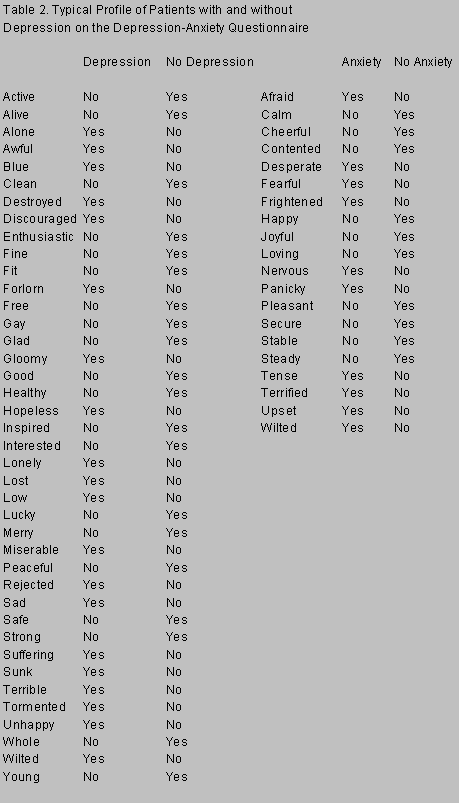
As an investigator who has participated in retrospective and prospective studies on depression in patients with PD, I believe the higher figure, 39%, is the more accurate. In 1979 my colleagues and I retrospectively analyzed PD patients and found depression in 25% of 168 demented PD patients and in 30% of 352 nondemented PD patients. Depression antedated PD in 19% of the depressed PD patients.19 Before their enrollment in a prospective study of the anti-parkinson drug, seligiline, 21 recently diagnosed, mildly disabled, nonlevodopa-treated, noncognitively impaired PD patients were evaluated by a psychiatrist using a structured interview, a depression rating scale, and a questionnaire that asked if particular words described the patients’ feelings during the previous month (Table 2).20 The mean age of the patients was 56 years, the mean duration of their PD was 1.6 years, and their mean disability as rated by the Hoehn and Yahr Scale was 1.3. Of these 21 patients, 9 (42%) were depressed.
Endogenous versus Exogenous Depression
The depression of PD is endogenous. It precedes the development of motor symptoms in 13 to 44% of patients 19,21,22,29 and is unrelated to the degree of physical disability8,22,24,31 or to the efficacy of antiparkinson treatment. Endogenous depression also may be complicated by exogenous depression. Exogenous depression is likely to occur in three types of patients: (1) patients with a parent, sibling, or close friend also disabled by PD; (2) patients undergoing a midlife crisis related to retirement, job loss, or a spouse’s illness; (3) or patients who suffer loss of self-esteem related to tremor or an autonomic nervous system (ANS) dysfunction such as impotence, drooling, or urinary incontinence. A skilled interviewer may separate the exogenous from the endogenous component of depression, and appropriate counseling may help resolve the exogenous component.
Autonomic Nervous System Dysfunction
Symptoms of ANS dysfunction, including impotence, drooling, urinary incontinence, excessive sweating, intolerance of heat and cold, and orthostatic hypotension, may complicate PD independent of depression and be unrelated to the severity of motor symptoms (Fig. 1). 1,9 The ANS symptoms, which are similar to those of the Shy-Drager syndrome, result from the degeneration of pigmented and nonpigmented noradrenergic and cholinergic neurons in the dorsal vagal nucleus, peripheral sympathetic chains, and sympathetic plexi of Meissner and Auerbach. 9 The degeneration of pigmented noradrenergic ANS neurons is associated with the formation of Lewy bodies. ANS symptoms such as drooling, orthostatic hypotension, and urinary incontinence may improve if treated with sympathomimetic drugs (e.g., midodrine, an alpha-1 agonist) or fluid regulation. Impotence may improve with counseling or medication.
Some ANS symptoms, such as excessive sweating or the intolerance of heat and cold, may be related to thyroid disease rather than to ANS dysfunction. When these symptoms are present, the thyroid should be evaluated. It is easier to correct symptoms arising from thyroid dysfunction than those arising from ANS dysfunction.
PD patients can experience trouble falling asleep, trouble staying asleep, and daytime drowsiness. 16,23 The sleep disorder may be a primary, endogenous disorder. It also may result from an inability to turn in bed, which is an early symptom of PD and one that may be independent of the severity of the disease (Fig. 2). The sleep disorder may include symptoms such as dystonic leg and arm cramps, restless legs, vivid dreams, hallucinations, agitation, and nocturnal hypervigilance alternating with daytime hypersomnolence. Typically, dystonic cramps and restless legs are related to a deficiency of dopamine. The other symptoms may be related to an excess of dopamine and serotonin. The antiparkinson drug levodopa and dopamine agonists can improve dystonic cramps and restless legs. In the order of their ability to provoke or aggravate the other symptoms, the anticholinergic agents are amantadine, seligiline, dopamine agonists, and levodopa.
The sleep disorder in untreated PD patients resembles that following von Economo’s encephalitis known as encephalitis lethargica or sleeping sickness . 11 Von Economo’s encephalitis appeared between 1917 and 1930 and then disappeared. Parkinsonism, a primary sleep disorder, depression, and a cognitive disorder or a combination of the four developed in approximately 70% of the patients who had von Economo’s encephalitis. 11 The parkinsonism, sleep disorder, depression, and cognitive disorder among postencephalitic parkinson patients and idiopathic PD patients were similar, reflecting the similarity of cortical and subcortical structures involved by the pathological processes of von Economo’s encephalitis and idiopathic PD.
The pathology of von Economo’s encephalitis consists of neuronal loss with neurofibrillary tangles among dopaminergic neurons of the substantia nigra, noradrenergic neurons of the locus ceruleus, serotonergic neurons of the dorsal raphé nucleus, cholinergic neurons of the nucleus basalis of Meynert, and neurons of the periaqueductal gray matter and thalamus. Many of these structures (i.e., the locus ceruleus, the dorsal raphé nucleus, the periaqueductal gray matter, and the thalamus) subserve sleep and wakefulness.
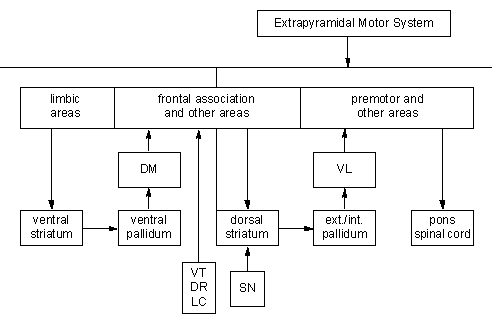
Figure 3. Overlapping anatomical substrates underlying the affective component of the cognitive disorder and the motor disorder associated with Parkinson’s disease. DM=dorsal median nucleus of the thalamus, VT=ventral tegmentum, DR=dorsal raphé nucleus, LC=locus ceruleus, SN=substantia nigra, and VL=ventrolateral thalamus.
Cognitive Disorder
A large but unknown number of patients develop a selective impairment in cognitive function. 2,4,6,17,18,25,27,33 This selective impairment in cognitive function, which reflects a global deterioration in cognitive function, is distinct from dementia. 2,4,6,17,18,25,27,33 Manifestations of the selective impairment in cognitive function without dementia may include slowness of information processing (usually referred to as bradykinesia) and altered executive functioning (e.g., planning, sequencing, innovating).
It may be difficult to distinguish a PD patient who has a selective impairment in cognition from a PD patient who is depressed. Both patients can exhibit behavioral changes such as declining job performance, erratic or inappropriate conduct, or an inability to accomplish previously learned tasks like balancing a checkbook or completing a crossword puzzle.
A selective impairment of cognition without dementia is thought to arise from damage to dopaminergic neurons in the medial portion of the substantia nigra, noradrenergic neurons in the locus ceruleus, serotonergic neurons in the dorsal raphé nucleus, and cholinergic neurons in the nucleus basalis of Meynert (Fig. 3). 14 The loss of neurons among PD patients who have a selective impairment in cognition overlaps the loss of neurons among PD patients who are depressed. The overlap accounts for the difficulty in distinguishing between the two groups.
The loss of dopaminergic, noradrenergic, serotonergic, and cholinergic neurons may disinhibit one or more nonmotor cortico-subcortical circuits including a dorsolateral prefrontal cortex-to-dorsolateral caudate nucleus-to-globus pallidus-to- ventroanterior and mediodorsal thalamic circuit, a lateral orbitofrontal cortex-to-ventromedial caudate nucleus-to-globus pallidus-to-ventroanterior and mediodorsal thalamic circuit, and an anterior cingulate gyrus-to-nucleus accumbens-to-globus pallidus-to-mediodorsal thalamic circuit. 11
The selective cognitive impairment without dementia among PD patients may be associated with affective symptoms distinct from depression, including anergy, apathy, anhedonia, and passivity. 4,17,20,27,33 Indeed, the term bradykinesia, now used to describe the slowness of information processing related to selective cognitive impairment without dementia, originally was used to describe patients with postencephalitic Parkinsonism who, in addition to exhibiting cognitive impairment without dementia, exhibited anergy, anhedonia, apathy, and passivity without depression. 25
In an undetermined subset of PD patients, depression may be a forerunner of dementia. 2,21,30 Whether selective impairment of cognition is a forerunner of dementia is unknown.
Treatment of Depression
For PD patients with exogenous depression, that is, for those with depression linked to having been diagnosed with PD or to other external situations in their life, counseling may be sufficient. For patients with a sustained depression, endogenous or exogenous, several approaches may be used. For some of these patients, counseling also may be sufficient; others can require treatment with antidepressants.
The selective serotonin reuptake inhibitors (SSRIs), fluoxetine, paroxetine, and sertraline, have become the mainstays of treatment. Unlike tricyclic antidepressants, SSRIs activate rather than sedate. This property may be desirable for patients who are anergic, apathetic, passive, and withdrawn, but it may be undesirable in patients who are agitated. In treating the depression of PD, the dose of SSRIs is similar to that administered to patients with nonPD depression: fluoxetine, 20 to 40 mg/day; paroxetine, 20 mg/day; and sertraline, 50 mg/day. Concern has been raised that the motor symptoms of PD can worsen during treatment with SSRIs. 13 This outcome, however, is unusual. Likewise, adverse interactions between SSRIs and selegiline occur but are rare. Nonetheless, patients should stop taking selegiline, if possible, when they start taking SSRIs.
Tricyclic antidepressants are another mainstay of treatment. Amitriptyline, nortriptyline, imipramine, desipramine, and doxepin vary in their ability to block the reuptake of noradrenaline and serotonin. They have different plasma half-lives and clearances. Their antidepressant, anticholinergic, and sedative properties are also different. Tricyclics can increase confusion or induce delirium in patients with cognitive impairment but no dementia. Consequently, the best choice is one with a short plasma half-life, rapid clearance, and little anticholinergic activity. The tricyclics, in decreasing order of their anticholinergic activity, are amitriptyline, imipramine, doxepin, desipramine, and nortriptyline.
Nortriptyline is the major metabolite of amitriptyline, and desipramine is the major metabolite of imipramine. Because nortriptyline and desipramine have less anticholinergic activity and are cleared more rapidly than their parent compounds, they are preferred for patients who are cognitively impaired. Nortriptyline is given in an initial night time dose of 20 to 40 mg. The initial night time dose of desipramine is 25 to 50 mg. The sedative properties of the tricyclics may be desirable in agitated patients but not in apathetic, passive, and withdrawn patients. In decreasing order of their sedative properties, the tricyclics are amitriptyline, doxepin, imipramine, desipramine, and nortriptyline.
Electroconvulsive therapy (ECT) may be used to treat depressed patients who have failed to improve with counseling and antidepressants. 10 A side benefit of ECT is that in some patients it may temporarily lessen the rigidity and bradykinesia of PD.
Treatment of Anxiety
Approximately 40% of PD patients are very anxious. 32 This anxiety may be a manifestation of depression. A nxiety may be a reaction to PD, or it may be part of PD and related to the loss of brain stem dopaminergic, noradrenergic, and serotonergic neurons with denervation of a limbic system-hypothalamic-striatal circuit.
Panic attacks, episodic outbursts of anxiety, are characterized by a variety of psychic, autonomic, and somatic symptoms, which include fear of dying, fear of going insane, breathlessness, diaphoresis, chest pain, choking, and dizziness. Panic attacks also can be a manifestation of depression. Panic attacks can simulate a heart attack, from which occasionally they must be differentiated.
In many PD patients, panic attacks are situationally cued and linked to immobility. 5 Consequently, among PD patients with diurnal fluctuations in performance, known as the “on-off” phenomenon, panic attacks occur during the “off” period. When panic attacks occur during the “off” period, their intensity parallels the difference in mobility between the “off” and the “on” periods. In these patients, treatment first should be directed toward decreasing the fluctuations before the panic attacks are treated. Some patients, however, experience panic attacks throughout the day, regardless of whether they are in an “on” or an “off” period. Anxiolytic medications are indicated for such patients or for those whose “off” periods fail to improve. Short-acting benzodiazepines—alprazolam and lorazepam—are the preferred medications. Typical doses of alprazolam are 0.5 to 1.0 mg three times a day while typical doses of lorazepam are 0.5 to 2.0 mg three times a day. Lower doses (by 50%) should be used to treat cognitively impaired patients. Clonazepam or the SSRIs also can be useful in treating panic attacks.
Acknowledgment
This analysis was supported, in part, by a grant from The Wallace Foundation, Carefree, Arizona.
References
- Appenzeller O, Goss JE: Autonomic deficits in Parkinson’s syndrome. Arch Neurol 24 :50-57, 1971
- Boyd JL, Cruickshank CA, Kenn CW: Cognitive impairment and dementia in Parkinson’s disease: A controlled study. Psych Med 21:911-921, 1991
- Brown RG, MacCarthy B, Gotham AM, et al: Depression and disability in Parkinson’s disease: A follow-up of 132 cases. Psychol Med 18:49-55, 1988
- Brown RG, Marsden CD: Cognitive function in Parkinson’s disease: From description to theory. Trends in Neuroscience 13:21-29, 1990
- Brown RG, Marsden CD, Quinn N, et al: Alterations in cognitive performance and affect-arousal state during fluctuations in motor function in Parkinson’s disease. J Neurol Neurosurg Psychiatry 47:454-465, 1984
- Cooper JA, Sagar HJ, Jordan N: Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability.Brain 114:2095-2122, 1991
- Cummings JL: Depression and Parkinson’s disease: A review. Am J Psychiatry 149:443-454, 1992
- Dakof GA, Mendelsohn GA: Parkinson’s disease: The psychological aspects of a chronic illness. Psychol Bull 99:375-387, 1986
- Den Hartog Jager WA, Bethlem J: The distribution of lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. J Neurol Neurosurg Psychiatry 23:283-289, 1960
- Faber R, Trimble M: Electroconvulsive therapy in Parkinson’s disease and other movement disorders. Mov Disord 6 :293-303, 1991
- Holt W: Epidemic encephalitis. Arch Neurol Psych 38 :135-144, 1937
- Jacobs DH, Shuren J, Bowers D, et al: Emotional facial imagery, perception, and expression in Parkinson’s disease. Neurology 45 :1696-1702, 1995
- Jansen Steur ENH: Increase of Parkinson disability after fluoxetine medication. Neurology 43 :211-213, 1993
- Jellinger K: Structural basis of dementia in Parkinson’s disease, in Korczyn AD (ed): Dementia in Parkinson’s Disease. Bologna, Italy: Monduzzi Editore SPA, 1994, pp 31-38
- Langston JW, Widner H, Goetz CG, et al: Core assessment program for intracerebral transplantations (CAPIT). Mov Disord 7:2-13, 1992
- Lees AJ, Blackburn NA, Campbell VL: The nighttime problems of Parkinson’s disease. Clin Neuropharmacol 11:512-519, 1988
- Lees AJ, Smith E: Cognitive deficits in the early stages of Parkinson’s disease. Brain 106:257-270, 1983
- Levin BE, Llabre MM, Weiner WJ: Cognitive impairments associated with early Parkinson’s disease. Neurology 39:557-561, 1989
- Lieberman A, Dziatolowski M, Kupersmith M, et al: Dementia in Parkinson disease. Ann Neurol 6:355-359, 1979
- Lieberman A, Hiesiger E: Selegiline as monotherapy in de novo Parkinson’s disease patients. BNI Quarterly 7:9-12, 1991
- Mayeux R, Stern Y, Rosen J, et al: Depression, intellectual impairment, and Parkinson disease. Neurology 31:645-650, 1981
- Mindham RH: Psychiatric symptoms in Parkinsonism. J Neurol Neurosurg Psychiatry 33:188-191, 1970
- Nausieda PA, Weiner WJ, Kaplan LR, et al: Sleep disruption in the course of chronic levodopa therapy: An early feature of the levodopa psychosis. Clin Neuropharmacol 5:183-194, 1982
- Riklan M, Levita E, Diller L: Psychologic studies in neurologic diseases. A review: Parkinson’s disease and multiple sclerosis. J Am Geriatric Soc 9 :857-867, 1961
- Rogers D: Bradyphrenia in parkinsonism: A historical review. Psychol Med 16 :257-265, 1986
- Ross ED, Rush AJ: Diagnosis and neuroanatomical correlates of depression in brain-damaged patients. Implications for a neurology of depression. Arch Gen Psychiatry 38 :1344-1354, 1981
- Saint-Cyr JA, Taylor AE, Lang AE: Neuropsychological and psychiatric side effects in the treatment of Parkinson’s disease.Neurology 43 :547-552, 1993
- Sano M, Stern Y, Williams J, et al: Coexisting dementia and depression in Parkinson’s disease. Arch Neurol 46 :1284-1286, 1989
- Santamaria J, Tolosa E, Valles A: Parkinson’s disease with depression: A possible subgroup of idiopathic parkinsonism.Neurology 36 :1130-1133, 1986
- Starkstein SE, Berthier ML, Bolduc PL, et al: Depression in patients with early versus late onset of Parkinson’s disease. Neurology 39 :1441-1445, 1989
- Starkstein SE, Robinson RG: Dementia of depression in Parkinson’s disease and stroke. J Nerv Ment Dis 179 :593-601, 1991
- Stein MB, Heuser IJ, Vade TW: Anxiety disorders in patients with Parkinson’s disease. Am J Psychiatry 147 :217-220, 1990
- Troster AI, Paolo AM, Lyons KE, et al: The influence of depression on cognition in Parkinson’s disease: A pattern of impairment distinguishable from Alzheimer’s disease. Neurology 45 :672-676, 1995
