Nonepileptic Seizures: Neuropsychological Mechanisms
Elizabeth W. Twamley, MA
Jennifer J. Bortz, PhD
Division of Neurology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
As many as 40% of patients evaluated at comprehensive epilepsy centers are diagnosed with nonepileptic seizures. Nonepileptic seizures resemble ictal events but are behavioral manifestations of psychological distress that lie outside the patient’s awareness and conscious control. Psychological trauma, closed head injury, family distress, and ineffective coping are key risk factors for the development of nonepileptic seizures. Neuroimaging studies of depression, anxiety, and somatization are helpful in understanding the biological substrates that may underlie nonepileptic seizures. Research suggests that disruptions in subcortical-frontal circuits reflect the neurophysiological underpinnings of nonepileptic seizures. These findings support hypotheses regarding frontal lobe dysfunction as a primary neuropsychological deficit in these patients. Increased understanding of the relationships between neurological mechanisms of nonepileptic seizures and psychological issues will ultimately improve treatment efficacy and enhance patients’ cognitive and emotional functioning.
Key Words: conversion reactions, epilepsy, mood disorders, nonepileptic seizures, posttraumatic stress disorders, seizures
Problems cannot be solved at the same level of awareness that created them. —Albert Einstein
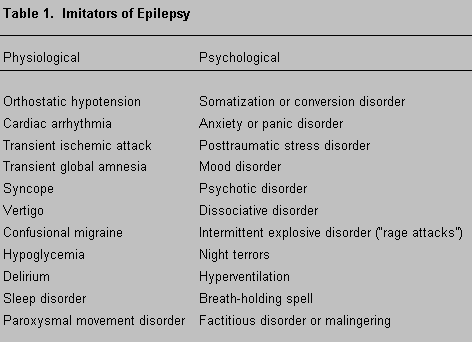
Epilepsy is a disorder marked by recurrent episodes of abnormal electrical discharges in the brain, which are accompanied by time-limited alterations in sensation, motor activity, behavior, or consciousness. In the absence of one sign—electroencephalographic (EEG) abnormality—many conditions are indistinguishable from ictal events. Consequently, differential diagnosis is an important but often challenging clinical task. Numerous physiologically and psychologically based conditions imitate epilepsy due to considerable overlap among the signs and symptoms of these disorders (Table 1).19,40 This article focuses on one of epilepsy’s most enigmatic imitators, nonepileptic seizures.
Terminology, Diagnosis, and Risk Factors of Nonepileptic Seizures
Nonepileptic seizures are behavioral and emotional manifestations of acute psychological distress that can resemble almost any seizure phenotype. Historically, these events have been called psychogenic seizures, pseudoseizures, pseudoepileptic seizures, hysterical seizures, functional seizures, and many other labels considered by many to be pejorative and confusing. At our center, we prefer to use the term episodic stress reaction in discussions with patients with nonepileptic seizures and their families to avoid communicating the notion that these events are not real or the equally erroneous impression that nonepileptic seizures are a seizure variant. Intractable nonepileptic seizures indeed represent a disabling disorder that affects individuals’ emotional well-being and cognitive efficiency, thereby creating excess disability in the form of financial, interpersonal, and social hardships. Patients with nonepileptic seizures often experience a considerable loss of independence, which can include the loss of employment and ability to drive.
Distinguishing patients with nonepileptic seizures from those with epileptic seizures often requires the collective efforts of specialists from the fields of neurology, neurophysiology, neuroradiology, psychiatry, and neuropsychology. No known clinical features are pathognomonic for nonepileptic seizures or can reliably differentiate these events from epileptic seizures.14 Videotelemetry studies, which permit behavioral events to be correlated with simultaneous video and EEG data, are often the only means of establishing a reliable diagnosis. Epilepsy specialty centers, which conduct intensive diagnostic and presurgical evaluations of patients with intractable seizures, report that as many as 40% of their patients are ultimately diagnosed with nonepileptic seizures. It is widely recognized that nonepileptic seizures and epileptic seizures can exist comorbidly. Between 6 and 40% of epilepsy patients have concomitant nonepileptic seizures38 while 12 to 36% of patients with nonepileptic seizures have concomitant epilepsy.28
Most patients with nonepileptic seizures are young adults with a history of severe psychological trauma. Approximately 75% are female.42 A history of trauma is a key risk factor. In a recent study, about 85% of patients with nonepileptic seizures reported childhood or adolescent sexual and/or physical abuse.7 Other studies, however, have reported a lower incidence (e.g., 32%) of such trauma.2 Differences in the prevalence of a history of trauma may reflect sampling errors or methodological differences in how clinical histories are elicited. However, the significance of trauma as a principal risk factor for nonepileptic seizures is indisputable.
Approximately 15% of patients with conversion disorders present with nonepileptic seizures as their primary symptom.30 Other psychiatric imitators of epilepsy include anxiety disorders, posttraumatic stress disorder, psychotic disorders, dissociative disorders, affective disorders, and personality disorders. Patients with nonepileptic seizures who do not have a conversion disorder are not predominantly female and are less likely to have a history of trauma.14
Traumatic head injury is another notable risk factor for nonepileptic seizures. Mild or minimal closed head injury, widely considered to carry no additional risk of epilepsy relative to the risk for the general population, has been shown to precipitate nonepileptic seizures. In a recent study,4 24% of patients with nonepileptic seizures had nonepileptic events attributed to their history of head injury. Of this group, 78% had sustained mild head injuries, but 22% had sustained moderate or severe head injuries. Eleven percent had histories of bona fide posttraumatic seizures, and 11% had histories of childhood seizures or febrile seizures.4 In another study,44 32% of nonepileptic seizure patients had a documented antecedent head injury, 91% of which were classified as minor. Of those who developed nonepileptic seizures after head injury, 12% also had a confirmed diagnosis of epilepsy. Perhaps head injury is sufficiently traumatizing in psychologically vulnerable individuals to produce a conversion disorder; premorbid psychiatric problems are common in mild head injury patients who develop nonepileptic seizures.4,44 Some patients have initial posttraumatic seizures and may later unconsciously adopt similar behavioral symptoms, especially when others in the patient’s environment attend to or otherwise reinforce sick-role behavior.
Nonepileptic Seizures in Other Populations
Recent research on nonepileptic seizures documents an increasing recognition of the syndrome in children, adolescents, and males. In children, the mean age of onset for nonepileptic seizures has been estimated at 12 years.37 The incidence of nonepileptic seizures in boys and girls is approximately equal before the age of 10 years, but its incidence in girls exceeds that in boys after age 12. Risk factors include family dysfunction and stress; children and adolescents who cannot live up to their parents’ expectations because of learning disabilities or other problems are also considered at risk.37 Among younger children, nonepileptic seizures are typically viewed as uncontrolled behavioral reactions to aversive stimuli rather than conversion reactions to trauma or stress.
The belief that nonepileptic seizures represent a uniquely “female” sickness is still widely held. Despite Charcot’s systematic research and extensive writings about conversion disorders in males more than 100 years ago,10 symptoms of conversion reactions in men often go unrecognized. About 25% of patients with nonepileptic seizures are men,42 and rates of childhood trauma in male patients with nonepileptic seizures appear markedly similar to those of women with nonepileptic seizures.7 In one study, males and females with nonepileptic seizures were equally likely to report histories of depression and substance abuse, but males presented with more frequent and more severe depression. Compared to their female counterparts, male patients with nonepileptic seizures also had higher frequencies of rage attacks, adolescent histories of conduct disorder, and antisocial behaviors.7
Approaches to Understanding Nonepileptic Seizures
Psychological Perspectives
Nonepileptic seizures are physical manifestations of extreme psychological distress, most often falling under the diagnostic umbrella of conversion reactions. The link between trauma and conversion disorders is well-documented.3 Theoretically, individuals with conversion disorders are believed to repress psychological distress precipitated by earlier trauma and to develop physical symptoms in response to subsequent environmental stress or internal conflict. In turn, the physical symptoms release underlying anxiety via alternative, nonpsychological pathways.22 Although secondary gain (e.g., nurturing or avoidance of aversive situations) may be derived from conversion symptoms, the development of true conversion symptoms is, by definition, unconscious and should not be confused with intentional faking of physical illness (i.e., malingering).
A history of abuse is associated with a higher incidence of somatic symptoms related to panic, depression, musculoskeletal pain, gastrointestinal disorders, and genito-urinary disorders.16,32 A recent study of female patients with fibromyalgia found that 57% reported a history of physical and/or sexual abuse and that those with a history of abuse perceived more pain and used more outpatient health care services and pain medication.1 A history of abuse is known to increase the likelihood of dissociation experiences,18 which are common in patients with nonepileptic seizures.
Somatoform disorders, including nonepileptic seizures, run in families and often represent a pattern of systemic distress. One recent study of patients with nonepileptic seizures and those with epileptic seizures showed that families of the former reported more health problems, distress, and criticism than families of the latter.45 Families of patients with nonepileptic seizures may not only experience more distress, but they may also have a narrow range of coping skills for dealing with such distress. Thus, in an environment with limited resources to cope effectively with stress or to otherwise resolve psychological dissonance, emotional tension may be expressed as physical signs and symptoms. Unconscious symptom modeling and reinforcement may also be common pathways linking dysfunctional coping mechanisms and the ultimate manifestation of conversion symptoms.
Biological Perspectives
Functional neuroimaging techniques, neurochemical discoveries, and other technological advances continue to broaden our understanding of biological mechanisms underlying psychiatric disorders. A Medline search restricted to the past 5 years elicited 500 articles referencing the terms “neurotransmitter” and “psychiatric,” and 860 articles were identified with the keywords “imaging” and “psychiatric.”
Within the realm of stress reactions, biochemical changes associated with excessive fluctuations in norepinephrine response have been closely linked to the intrusive memory experiences of trauma patients diagnosed with posttraumatic stress disorder.26 The endogenous production of opioids, triggered by stress and linked to norepinephrine regulation, appears to produce dissociative “numbing” of emotional and physical reactivity among these patients. Dissociation in patients with nonepileptic seizures may likewise result from sensory inhibition related to unconscious, intrusive trauma or other stress-related memories processed in subcortical neural networks.26 Anecdotally, individuals with nonepileptic seizures seldom report emotional triggers connected to their spells, suggesting that the numbing reaction may occur even before an emotional trigger can be perceived and identified consciously.
In a related fashion, the hypothalamic-pituitary-adrenal axis has been implicated in somatization disorders. Chronically and acutely stressed individuals who develop stress-related bodily disorders demonstrate low adrenal activity and decreased cortisol responses,23 suggesting a link between suboptimal exposure to the protective effects of cortisol and the manifestation of somatization behaviors. Empirical research has not determined the direction of the effect, and further work is needed to clarify the nature and implications of these data. Such findings, however, provide preliminary evidence of a biological substrate for somatization behaviors.
Neuroscience research has experienced considerable growth, fostered by quantitative measures of regional cerebral blood flow and glucose metabolism via positron emission tomography (PET) and functional magnetic resonance (fMR) imaging technologies. Numerous PET studies have documented frontal lobe hypometabolism in patients with anxiety disorders, schizophrenia, and mood disorders. Of particular interest is a line of research indicating differential alterations in the resting levels of cerebral blood flow and glucose metabolism in individuals at various phases of major depression and treatment intervention.15 This work has provided quantitative indices of the severity of depression and the efficacy of treatment.
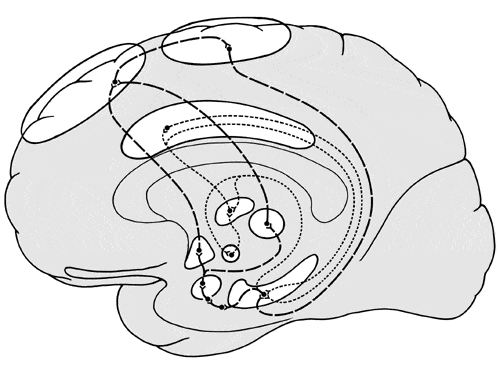
Decades after Papez’s seminal description of the neural circuitry involved in emotional processing, PET studies have also provided empirical evidence of unique anatomical pathways involved in primary mood disorders. In their review, Kennedy and colleagues27 synthesized findings from animal, clinical, and functional imaging studies, implicating distinct excitatory and inhibitory neural pathways associated with depressive disorders (Fig. 1). These authors posit that amygdalar hypoactivity would disrupt prefrontal regions associated with conscious emotional experience. Following this line of reasoning, this effect could explain neural substrates of memory lapses, dissociative experiences, and other alterations in consciousness experienced by patients with nonepileptic seizures.
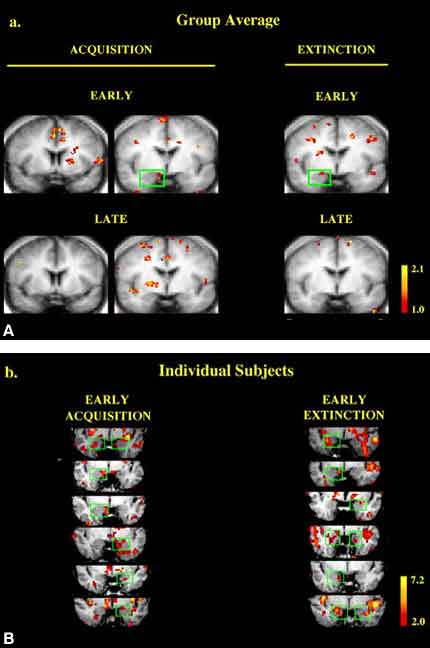
Similarly, fMR imaging has been used to investigate combined functional and structural mechanisms of the central nervous system underlying psychiatric disorders. Such studies have investigated corticostriatal and limbic circuitry associated with obsessive-compulsive disorder,8 activation of the posterior cingulate in response to threat-related stimuli,33 and disruptions in subcortical neural structures and pathways associated with primary mood and anxiety disorders.27,41 For example, LaBar and colleagues29 recently examined the contribution of amygdala activation to acquisition and extinction processes involved in emotional learning. Eighteen normal subjects were randomly exposed to visual cues preceding a shock stimulus (CS+) and to a second visual cue presented alone (CS-). Serial coronal fMR images of the amygdala were acquired during stimulus presentation. The amygdala and periamygdaloid cortex were activated during conditioned fear acquisition (CS-) and extinction phases (Fig. 2). Autonomic indices of conditioning also correlated positively with activation during the acquisition phase, and a temporal gradient of the amygdalar response was documented during extinction trials. These data provide further evidence of neural networks underlying psychiatric disorders and may define a mechanism by which anxiety symptoms associated with nonepileptic seizures result from neuronal activation secondary to a conditioned fear response.
Together, these data provide preliminary evidence of both persistent and potentially reversible neuropsychological dysfunction in primary brain structures and function associated with clinical and behavioral features of nonepileptic seizures. To date, no neuroimaging studies of nonepileptic seizures have been published. However, neuroimaging studies of anxiety, somatization, and depression provide promising leads for future research regarding the biological substrates of nonepileptic seizures. Undoubtedly, diagnostic and treatment challenges inherent in this disorder as well as associated financial and social costs will propel work in this area.
Neuropsychological Profiles of Patients with Nonepileptic Seizures
Patients with nonepileptic seizures report significant restrictions in cognitive functions, but their neuropsychological profiles consistently have been indistinguishable from those of patients with well-defined epileptic seizures.24 Binder and colleagues5 compared the performance of 30 patients with nonepileptic seizures, 42 patients with epileptic seizures, and 47 control subjects on a comprehensive battery of neuropsychological tests. Compared to the healthy control subjects, the performance of the groups with nonepileptic and epileptic seizures was impaired on 10 of 13 tests. Posthoc comparisons revealed no differences between the groups with nonepileptic and epileptic seizures on tests that assessed intellectual ability, motor speed and coordination, attention, problem-solving, and executive functioning.
An investigation of verbal learning and memory likewise found no mean differences in the initial acquisition of information, the rate of learning over repeated trials, or delayed recall of a 16-item word list between patients with nonepileptic seizures and those with epileptic seizures.6 Group differences emerged only in qualitative analysis of patients’ performance on recognition memory tests. Specifically, patients with nonepileptic seizures exhibited a negative response bias and patients with dominant temporal seizure foci produced a positive response bias. The findings of verbal memory deficits in patients with nonepileptic seizures parallel findings of decreased auditory attention and verbal working memory in adult survivors of severe childhood abuse.9
Objective measures of personality have not been particularly helpful in the differential diagnosis of nonepileptic seizures. For example, no single profile type on the Minnesota Multiphasic Personality Inventory (MMPI) or MMPI-2 differentiates groups with nonepileptic seizures and epileptic seizures.25 Some studies, however, have shown MMPI/MMPI-2 Scale 3 elevations to be more common in patients with nonepileptic seizures than in patients with epileptic seizures.12,13,35 A recent study5 found that personality variables and test-taking effort (as measured by the MMPI-2 and the Portland Digit Recognition Test) correctly classified 80% of the patients with nonepileptic seizures and epileptic seizures. Although certain personality patterns are likely to predominate among patients with nonepileptic seizures, the constructs of the MMPI/MMPI-2 alone are insufficient to differentiate the two groups.
Mechanisms of Neuropsychological Impairment in Nonepileptic Seizures
The question of whether cognitive deficits associated with nonepileptic seizures are the sole product of emotional reactivity or an organic dysfunction has been a topic of considerable debate. Increasingly, ongoing theoretical discussions and empirical data appear to blur this distinction. Recent research has focused on investigating the nature of biological deficits in psychiatric disorders, be they structural or functional and reversible or irreversible.
Several lines of reasoning converge to suggest that the cognitive difficulties of patients with nonepileptic seizures are associated with functional inefficiencies in executive systems (i.e., frontal lobe) processing. Comorbid depression and anxiety contribute to neuropsychological impairments in attention, concentration, memory, and speed of information processing.39 This constellation of cognitive deficits is often associated with diminished integrity of frontal lobe functions. Similarly, individuals diagnosed with posttraumatic stress disorder often demonstrate limitations in sustained attention, complex attentional processing, initial acquisition of information, and behavioral inhibition.43 This pattern of neuropsychological performance deficits again suggests functional compromise of frontal systems in distressed patients. Furthermore, such deficits may disrupt encoding processes, resulting in a degree of memory impairment comparable to that seen in patients who have sustained severe neurological insults.11
In terms of sustained attention and effortful processing abilities, patients with nonepileptic seizures simply may not have the necessary emotional or biological resources to perform successfully on challenging neuropsychological tasks. Ellis’s and Ashbrook’s17 resource allocation model of attention further illuminates the mechanisms that may underlie the neuropsychological deficits of patients with nonepileptic seizures. According to this model, the brain has a finite amount of attentional resources to expend at any given time. When emotional demands deplete this store acutely or chronically, the brain is left with fewer and less efficient means to handle higher-order cognitive tasks.
Functional neuroimaging studies of depression shed light on the mechanisms underlying executive systems dysfunction in distressed patients. A consistent finding is disruption of paralimbic-cingulate-prefrontal pathways, leading to frontal lobe hypometabolism.20 Structural neuroimaging studies have also revealed anatomic abnormalities associated with depression. The most consistent structural correlate is an increased number of subcortical white matter and periventricular hyperintensities in the brains of depressed subjects.41 A recent case report suggests that extreme environmental stress may precipitate neuronal death in regions of the brain that subserve memory functions (e.g., hippocampus).34 Additional research is needed to establish the frequency with which permanent cell loss occurs as a result of psychological stressors.
Future research may confirm that patients with nonepileptic seizures exhibit divergent patterns of neuropsychological performance, depending on whether depression or anxiety is the manifestation of distress. Depressed patients with nonepileptic seizures might exhibit difficulties linked to hypoarousal, whereas anxious patients with nonepileptic seizures might exhibit difficulties linked to hyperarousal. In both cases, frontal lobe functions and subcortical-frontal pathways are implicated in the neurophysiology of nonepileptic seizures. These same pathways may affect the ability of patients with nonepileptic seizures to control arousal levels subconsciously and to inhibit dissociative responses and intrusive memories.
Treatment of Nonepileptic Seizures
Surprisingly little is known about the efficacy of treatment in this patient population. In one study, a favorable prognosis was associated with female gender, higher intelligence, normal EEG findings, psychosocial independence, and the absence of a psychiatric history. Unemployment, family history of epilepsy, comorbidity of other psychiatric disorders, and chronicity of nonepileptic seizures have been associated with poor therapeutic outcomes.31,36 In two studies, full remission of symptoms was documented in 40% and 34% of subjects, respectively. Unfortunately, no empirical studies of the differential efficacy of various treatment approaches in this population have been published.
Conclusions
Conversion processes, acute stress, somatization tendencies, and anxiety and mood disorders have all been implicated as factors that contribute to nonepileptic seizures. The common underlying factor appears to be emotional distress, and it has been proposed that the body expresses pschological conflict when the individual is unable to communicate distress effectively.21 Inability to resolve underlying conflicts or the reinforcement of nonepileptic seizures may serve to maintain the symptoms. Despite considerable knowledge about the risk factors for nonepileptic seizures, the mechanisms whereby symptoms are unintentionally produced remain uncertain. This process is understood to occur on an unconscious level and may be mediated by biological factors such as neuroendocrine functioning, neurotransmitter responses, and structural abnormalities of the brain. Future research investigating the links among emotional functioning, dissociation, and inhibitory processes may identify neurophysiological contributions to nonepileptic seizures. Such research may be helpful in fostering the development of effective treatments for patients with nonepileptic seizures.
References
- Alexander RW, Bradley LA, Alarcon GS, et al: Sexual and physical abuse in women with fibromyalgia: Association with outpatient health care utilization and pain medication usage. Arthritis Care Res 11:102-115, 1998
- Alper K, Devinsky O, Perrine K, et al: Nonepileptic seizures and childhood sexual and physical abuse. Neurology 43:1950-1953, 1993
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders. Washington, D.C.: American Psychiatric Association, 1994
- Barry E, Krumholz A, Bergey GK, et al: Nonepileptic posttraumatic seizures. Epilepsia 39:427-431, 1998
- Binder LM, Kindermann SS, Heaton RK, et al: Neuropsychologic impairment in patients with nonepileptic seizures. Arch Clin Neuropsychol 13:513-522, 1998
- Bortz JJ, Prigatano GP, Blum D, et al: Differential response characteristics in nonepileptic and epileptic seizure patients on a test of verbal learning and memory. Neurology 45:2029-2034, 1995
- Bortz JJ, Stonnington C: Clinical and neuropsychological features of male patients with nonepileptic seizures. Epilepsia 36:136, 1995
- Breiter HC, Rauch SL: Functional MRI and the study of OCD: From symptom provocation to cognitive-behavioral probes of cortico-striatal systems and the amygdala. Neuroimage 4:S127-S138, 1996
- Bremner JD, Randall P, Scott TM, et al: Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Res 59:97-107, 1995
- Micale MS: Charcot and the idea of hysteria in the male: Gender, mental science, and medical diagnosis in late nineteenth-century France. Medical History 34:363-411, 1990
- Christensen H, Griffiths K, Mackinnon A, et al: A quantitative review of cognitive deficits in depression and Azheimer-type dementia. J Int Neuropsychol Soc 3:631-651, 1997
- Connell BE, Wilner AM: MMPI-2 distinguishes intractable epilepsy from pseudoseizures: A replication. Epilepsia 37:19, 1996
- Derry PA, McLachlan RS: The MMPI-2 as an adjunct to the diagnosis of pseudoseizures. Seizure 5:35-40, 1996
- Devinsky O: Nonepileptic psychogenic seizures: Quagmires of pathophysiology, diagnosis, and treatment. Epilepsia 39:458-462, 1998
- Drevets WC: Functional neuroimaging studies of depression: The anatomy of melancholia. Annu Rev Med 49:341-361, 1998
- Drossman DA: Irritable bowel syndrome and sexual/physical abuse history. Eur J Gastroenterol Hepatol 9:327-330, 1997
- Ellis HC, Ashbrook PW: The “state” of mood and memory research: A selective review. J Social Behavior and Personality 4:1-21, 1989
- Farley M, Keaney JC: Physical symptoms, somatization, and dissociation in women survivors of childhood sexual assault. Women Health 25:33-45, 1997
- Fisher RS: Imitators of Epilepsy. New York: Demos Publications, 1994
- Goodwin GM: Neuropsychological and neuroimaging evidence for the involvement of the frontal lobes in depression. J Psychopharmacol 11:115-122, 1997
- Griffith JL, Griffith ME: The Body Speaks: Therapeutic Diaglogues for Mind-Body Problems. New York: Basic Books, 1994
- Griffith JL, Polles A, Griffith ME: Pseudoseizures, families, and unspeakable dilemmas. Psychosomatics 39:144-153, 1998
- Heim C, Ehlert U, Hanker JP, et al: Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosom Med 60:309-318, 1998
- Hermann BP: Neuropsychological assessment in the diagnosis of non-epileptic seizures, in Rowan AJ, Gates JR (eds): Non-Epileptic Seizures. Stoneham, MA: Butterworth-Heinemann, 1993, pp 221-232
- Kalogjera-Sackellares D, Sackellares JC: Analysis of MMPI patterns in patients with psychogenic pseudoseizures. Seizure 6:419-427, 1997
- Katz L, Fleisher W, Kjernisted K, et al: A review of the psychobiology and pharmacotherapy of posttraumatic stress disorder. Can J Psychiatry 41:233-238, 1996
- Kennedy SH, Javanmard M, Vaccarino FJ: A review of functional neuroimaging in mood disorders: Positron emission tomography and depression. Can J Psychiatry 42:467-475, 1997
- Kuyk J, Leijten F, Meinardi H, et al: The diagnosis of psychogenic non-epileptic seizures: A review. Seizure 6:243-253, 1997
- LaBar KS, Gatenby JC, Gore JC, et al: Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron 20:937-945, 1998
- Leahey T, Harris RJ: Fundamentals of conditioning, in Leahey T, Harris RJ (eds): Human Learning. Englewood Cliffs: Prentice Hall, 1985, pp 21-42
- Lempert T, Schmidt D: Natural history and outcome of psychogenic seizures: A clinical study in 50 patients. J Neurol 237:35-38, 1990
- Lesserman J, Li Z, Drossman DA, et al: Selected symptoms associated with sexual and physical abuse history among female patients with gastrointestinal disorders: The impact on subsequent health care visits. Psychol Med 28:417-425, 1998
- Maddock RJ, Buonocore MH: Activation of left posterior cingulate gyrus by the auditory presentation of threat-related words: An fMRI study. Psychiatry Res 75:1-14, 1997
- Markowitsch HJ, Kessler J, Van Der Ven C, et al: Psychic trauma causing grossly reduced brain metabolism and cognitive deterioration. Neuropsychologia 36:77-82, 1998
- Mason SL, Mercer K, Risse GL, et al: Clinical utility of the MMPI-2 in the diagnosis of non-epileptic seizures. Epilepsia 37:18, 1996
- Meierkord H, Will B, Fish D, et al: The clinical features and prognosis of pseudoseizures diagnosed using video-EEG telemetry. Neurology 41:1643-1646, 1991
- Metrick ME, Ritter FJ, Gates JR, et al: Nonepileptic events in childhood. Epilepsia 32:322-328, 1991
- Ramsay RE, Cohen A, Brown MC: Co-existing epilepsy and non-epileptic seizures, in Rowan AJ, Gates JR (eds): Non-Epileptic Seizures. Stoneham, MA: Butterworth-Heinemann, 1993, pp 47-54
- Reitan RM, Wolfson D: Emotional disturbances and their interaction with neuropsychological deficits. Neuropsychol Rev 7:3-19, 1997
- Rowan AJ, Gates JR: Non-Epileptic Seizures. Stoneham, MA: Butterworth-Heinemann, 1993
- Soares JC, Mann JJ: The anatomy of mood disorders—review of structural neuroimaging studies. Biol Psychiatry 41:86-106, 1997
- Van Merode T, De Krom MC, Knottnerus JA: Gender-related differences in non-epileptic attacks: A study of patients’ cases in the literature. Seizure 6:311-316, 1997
- Vasterling JJ, Brailey K, Constans JI, et al: Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology 12:125-133, 1998
- Westbrook LE, Devinsky O, Geocadin R: Nonepileptic seizures after head injury. Epilepsia 39:978-982, 1998
- Wood BL, McDaniel S, Burchfiel K, et al: Factors distinguishing families of patients with psychogenic seizures from families of patients with epilepsy. Epilepsia 39:432-437, 1998