
Surgical Treatment of Intractable Epilepsy
Kris A. Smith, MD
Paul W. Detwiler, MS, MD
Randall W. Porter, MD
Division of Neurological Surgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
Surgery for medically intractable epilepsy remains an underutilized treatment. The purpose of this article is to inform readers of the surgical treatments available for intractable seizures and to stress their safety and efficacy when the medical advances of the past decade are incorporated into the procedures.
Key Words: cortical resection, epilepsy, selective amygdalohippocampectomy, seizure, temporal lobectomy
The surgical treatment of epilepsy was pioneered by Penfield, Jasper, Rasmussen, and colleagues in Montreal and by Falconer and colleagues in London in the 1930s.5-9,44-46 Considering the limited understanding of the condition at the time, their clinical outcomes were remarkably good. The development of current technology such as high-resolution magnetic resonance (MR) imaging and positron emission tomography (PET), microneurosurgical techniques, neuroanesthesia, frameless stereotactic technology, digital electroencephalography (EEG), brain mapping, functional MR imaging, and magnetoencephalography (MEG) has helped make the surgical evaluation and treatment of intractable epilepsy safer, more effective, and applicable to more patients than the early surgical pioneers could have imagined.4,9,16,25,28,51,53,54,56,58
Approximately 0.5% to 1% of the population suffer from some type of epilepsy.4,9,16,25,28,51,53,54,56,58 About 70% are well controlled with medical therapy, leaving 30% who are not. About half of the patients intractable to pharmacological treatment could either be controlled medically or cured with surgical treatment. It has been estimated that 100,000 people in the United States would benefit from epilepsy surgery.4 In contrast, approximately 2000 procedures are performed annually. The acceptance and availability of this therapy are increasing, but it is still underutilized. Furthermore, surgical treatment tends to be offered only in specialized centers.
Surgery is underutilized for several reasons.4 First, patients and their families are unaware of the option of surgical treatment. If presented with the option, they are often misinformed about the safety and effectiveness of contemporary procedures. Second, patients are usually unemployed as a result of their epilepsy and have limited access to specialized medical care. Furthermore, payers limit access to specialized centers capable of offering surgical treatment. Third, primary care physicians and general neurologists still tend to have an aversion to referring epilepsy patients for surgical evaluation. This reluctance may also partially reflect socioeconomic-insurance issues and incentives against superspecialty referrals. Unfortunately, much of the reluctance may reflect a lack of knowledge about the available techniques. The purpose of this article is to inform readers of the available surgical treatments for intractable seizures and to stress their safety and efficacy when the medical advances of the past decade are incorporated into the procedures.
Patient Evaluation
The evaluation and treatment of patients with refractory seizures are team efforts that require the expertise of specialized neurologists, neurosurgeons, neuropsychologists, and neuroradiologists. The most important aspects of evaluating patients with epilepsy for the possibility of undergoing surgical therapy are diagnosing intractable seizures accurately and localizing the anatomical origin of the seizure onset, also known as the epileptic focus.10,25,40,51,53,54,58,63 Before they are considered for surgical treatment, all patients must have failed medical therapy. Several anticonvulsant medications at documented therapeutic levels must be tried before the determination of medically intractable seizures is made. A thorough medical history of the patient, including observations by friends and family members, is paramount. A reliable observer’s description is necessary because patients are often unaware of many or all of their seizures and therefore are unable to provide an accurate history. Outpatient EEG is seldom helpful because a normal interictal EEG does not exclude patients as potential candidates and an abnormal EEG does not adequately localize or characterize their seizures.15
The hallmark of the preoperative evaluation is digital video-EEG telemetry.51 Patients are monitored in an epilepsy monitoring unit. Under controlled conditions, with a standardized array of scalp electrodes in place, the patient is allowed to have several seizures, which are digitally recorded for analysis. The electrical onset of the seizures (EEG) is then accurately compared to the clinical onset of the seizure (video) event. Many patients who are believed to have uncontrolled epilepsy have pseudoseizures. This diagnosis is ruled out with confidence when normal EEG patterns are documented in the presence of the bizarre behavior associated with pseudoseizures. Conversely, many patients who are thought to have pseudoseizures, even by experienced epileptologists, are found to have true epilepsy when abnormal EEG patterns are found to correlate with the bizarre behavior.
Modern high-resolution neuroimaging is of utmost importance in the evaluation of epilepsy patients.25,40,49,53,59 MR imaging is the most important modality, but even it cannot detect all relevant conditions. For example, high-quality standard MR imaging does not detect mesial temporal sclerosis (MTS), the most common surgically treatable cause of refractory seizures in temporal lobe epilepsy. MTS, however, can be diagnosed in most patients by a special epilepsy protocol that includes thin, symmetrical, coronal slices through both temporal lobes. Another common cause of refractory seizures is cortical dysplasia or heterotopias. These benign lesions are routinely missed on standard MR imaging studies but easily seen on specialized sequences designed to disclose these abnormalities. Fortunately, when areas of cortical dysplasia or heterotopias are removed microsurgically, the patient is unlikely to incur a neurological deficit and is likely to be cured of epilepsy.
Cavernous malformations are benign vascular lesions that can cause epilepsy. They are easily detected by MR imaging and usually safely removed surgically.25 Many other relatively rare benign tumors and occasionally malignant tumors can be detected by MR imaging and are potential causes of intractable seizures. Therefore, patients with long-standing epilepsy who have not undergone a recent specialized MR imaging epilepsy protocol should be imaged with these new techniques to rule out a potentially curable lesion.
PET is also valuable for localizing seizure foci.4,40 It is rare to obtain images during an ictal event when the seizure focus would appear as a discrete spot of hyperactivity. Such a finding, however, is useful when it occurs. Most PET images are obtained at baseline interictal states, and regions of epileptogenic tissue appear as areas of relative hypometabolism. Most patients with temporal lobe epilepsy (70 to 80%) have unilateral temporal hypometabolism on the side of seizure onset. Patients with unilateral temporal hypometabolism on PET, an MR image study consistent with MTS, and lateralizing EEG findings are excellent candidates for surgical resection of the mesial temporal lobe and have an 85% chance of being rendered seizure free as a result of the operation.40,59 PET is also useful for excluding patients who are not likely to benefit from surgery. Although a nonlateralizing PET does not preclude a good surgical outcome, the presence of bilateral temporal hypometabolism is suggestive of bilateral seizure onset and the patient’s chance of improving significantly after resection is poor.
Single-photon emission computed tomography (SPECT) and MEG are not part of the standard evaluation of surgical candidates, but these modalities may be valuable in specific cases. The most challenging patients with uncontrolled epilepsy to treat are those with nonlesional, extratemporal foci. Recent reports suggest that localization with ictal SPECT is promising, especially when co-registered with MR images of these patients.30,32 A special technique of subtracting interictal SPECT images from ictal SPECT studies has proven value for localization with good surgical results.
Functional MR imaging53 is a new and exciting technology that permits testing paradigms to be performed by the patient while in the scanner. The MR imaging signals can be processed to detect microalterations in blood flow to areas of the brain activated during the testing paradigm. Functional areas can thereby be identified in individual patients.
Neuropsychiatric testing is also part of the standard preoperative evaluation for epilepsy.2,13,15,18,19,31,36,59,60,62 This evaluation has value for both localization and prognosis. Typically, patients with temporal lobe epilepsy have lateralizing findings on detailed neuropsychiatric testing. This information can confirm the side of a seizure-producing lesion. Patients with left temporal seizures, for example, tend to have language and verbal memory deficits, whereas those with right temporal lobe seizures tend to have impairments of visuospatial memory. Furthermore, it is useful to know the extent of the impairments before surgery so that patients can be counseled about functional expectations after surgery. Most patients’ impairments do not increase after resection. In fact, many improve during their long-term follow-up if they are rendered free of seizures.
The Wada test is an important preoperative evaluation for all patients who are strong candidates for temporal lobe resections.15,59 The test involves angiographic placement of a catheter in the internal carotid artery (ICA) on the planned side of resection. Amobarbital is selectively injected into the ICA while the patient is awake and monitored by clinical examination and EEG. The initial intent of the Wada test was to document the hemisphere of language dominance. This information was important because larger areas of the temporal lobe could then be resected in the nonspeech-dominant hemisphere.
Today, resections are standardized on both sides. As discussed later, the recent trend is to perform selective mesial temporal lobe resections with no resection of the lateral cortex. Therefore, the side of language dominance is less important when surgical resection is being considered. Today, the main reason for subjecting a patient to a Wada test is to elucidate whether the patient’s memory function will be adequate with only one remaining hippocampus. Thus, a resection is pharmacologically but temporarily simulated. The patient is then tested for object and word memory in a variety of tasks and scored to assess unilateral memory function. If the patient’s memory is adequate on the side of the proposed resection during the Wada test, the surgeon can proceed confidently with an aggressive hippocampal resection and hope to cure the patient’s seizures with minimal risk of an iatrogenic neurological deficit.
Invasive Recording Techniques
As a result of the multimodality testing as outlined above, the seizure focus of most patients is localized satisfactorily, and invasive monitoring is unnecessary before resection. In a small subgroup of patients, however, the data regarding the location or consistency of seizure onset may be contradictory or insufficient. Such patients must then have depth electrodes or subdural grid electrodes implanted to ascertain the area and extent of resection required.3,10,34,51,54,58
Depth Wires
Depth electrodes are small diameter silastic catheters with electrode contacts spaced at even intervals.34,51,58 There are usually eight contacts per wire. The catheters are inserted with a rigid stylet under stereotactic guidance to ensure optimal and safe positioning. After the stylet is removed, the soft catheter poses a minimal risk of injury even if the brain shifts or moves, which can occur during a seizure. The depth wires are secured to the skull with a bolt that also serves as a seal to prevent cerebrospinal fluid leaks. The insertion requires only a tiny scalp incision large enough to make a twist drill opening.
Because the electrode contacts are located within the brain tissue, it is possible to obtain precise recordings without movement or muscle artifacts. Typically, the earliest onset of the seizures is detected electrically before the patient is aware of an aura or seizure. Depth wires are usually implanted to confirm that patients are candidates for mesial temporal resection or to rule out bilateral temporal seizures. The standard array used at our institution consists of eight electrode wires positioned bilaterally in the amygdala, hippocampus, orbitofrontal cortex, and supplementary motor cortex. In most patients with suspected temporal lobe epilepsy, seizures recorded with this array will determine whether they are candidates for resection.
Depth wires are seldom useful for evaluating patients with superficial cortical lesions. Depth wires do not sample large cortical areas but rather give highly sensitive recordings from specific areas of suspected seizure onset.58 Therefore, depth wires should not be thought of as tools for a “fishing expedition.” Rather, they are tools for confirmatory testing of a suspected site of seizure onset based on existing information. The use of depth wires is associated with a 2% risk of intracerebral or subarachnoid hemorrhage and about a 1% incidence of infection.
Subdural Grid Electrodes
When a seizure focus is suspected to be in a cortical area, with or without an associated lesion, the most accurate way of determining that the seizures actually originate from the suspected area is by recording directly from the cortex with implanted subdural grid electrodes.34,54,58 The electrodes are flat, disc-shaped contacts made of platinum-iridium embedded in flat sheets of flexible silastic. Various sizes are available, and all can be cut and tailored for individual patients. The largest grid is an 8 x 8 square with a total of 64 contacts, each about 1 cm apart. Many sizes and configurations and combinations of different grids are routinely used to enable a complete investigation of an individual patient. Most grids require a craniotomy and large dural opening for placement under direct vision.
We routinely use a frameless stereotactic guidance system (ISG Technologies, Mississauga, Ontario, Canada) to help place the craniotomy and the grids over the area of interest.16 In our case, the ISG Wand can be re-registered to internal landmarks, typically on the skull around the craniotomy site for later use in guiding the resection. The individual contact points in the grid are numbered, and they also can be registered in the stereotactic space for later reference during the resection.
The grid electrodes serve two functions. The first function is to record the area of seizure onset. It is important to have generous electrode coverage around the area of suspicion to avoid the unfortunate circumstance of the seizures first being detected on the edge of the grid. If this situation occurs, one is still uncertain whether the seizure originated at the edge of the grid or at a distal point and its spread was simply first detected at the edge. Even if the seizure originated from the cortex under the electrodes located at the edge, one would not know how much cortex to resect beyond the edge.
Second, grid electrodes are used for functional brain mapping.3,10 After the grid electrodes have been implanted and the patient has recovered from anesthesia, the external wires can be connected to an impulse generator for bipolar stimulation. The neurologist and/or neuropsychologist can perform various testing paradigms by stimulating the electrodes sequentially. During stimulation of a specific electrode pair, alterations in the performance of a task indicate that the cortex beneath that electrode pair is necessary for the function tested. When completed, the stimulation and testing paradigms generate an accurate, anatomically correlated, functional brain map of the cortex under the grid. Often, the stimulation of certain cortical areas, even at low currents, results in the onset of a seizure. If consistent, this low-threshold of epileptogenesis in certain areas can be useful for determining the areas of seizure onset. With the results of brain mapping, the surgeon can resect the cortical areas where the seizures arise and confidently avoid areas known to serve eloquent functions. Such mapping procedures are necessary and performed routinely when the areas of seizure onset are near language and primary motor or sensory areas of the brain.
Subdural Strip Electrodes
Subdural strip electrodes are just like grid electrodes except that they consist of a single or double row of electrodes in lengths of 4 to 8 cm.34 Because of their small width, they can be positioned through small burr holes rather than through craniotomies. Some centers use bilateral subtemporal strips as an alternative to depth wires to evaluate suspected temporal lobe epilepsy. The disadvantage of this technique, however, is that the parahippocampal gyrus is recorded directly, but the hippocampus is not. Theoretically, at least, a seizure could spread contralaterally before it is detected and thus result in false lateralization. Because depth electrodes record from within the hippocampus directly and require smaller incisions to implant, we prefer depth wires to subtemporal strips for patients with temporal lobe epilepsy. Multiple strips can be implanted in many different directions from bilateral burr holes to permit large cortical areas to be sampled and cranial access to be minimized. This approach is quite useful for ruling out multifocal epilepsy when no lesion is present.
Epidural Electrodes
Grid electrodes can also be placed in the epidural space to improve recording compared to scalp electrodes.40 Localization, however, is less accurate than with subdural electrodes, and mapping can produce patient discomfort during dural stimulation. Epidural grids are useful in patients suspected of having an epileptogenic region under a prior craniotomy, where dissecting the dura off the brain is both tedious and potentially damaging. Single percutaneous epidural electrodes (PEGs) can also be implanted through small twist drill holes in multiple locations. PEGs are used as an adjunct to rule out a seizure focus outside the area of primary suspicion. No stimulation or mapping can be obtained with PEGs. However, recording quality is superior to scalp electrodes.
Procedures for Surgical Resection
Temporal Lobectomy
The most common surgical procedure used to treat intractable temporal lobe seizures is temporal lobectomy.1,9,10,20,21,34,40,43 Since its introduction by Penfield, multiple variations and modifications of temporal lobectomy have been developed. Early surgeons such as Penfield, Forester, Green, and others thought that intraoperative electrocorticography (EcoG) was important in determining the extent of the resection needed to control a patient’s seizures.40 Some centers still rely on EcoG. Today, however, studies have found no correlation between the presence of intraoperative spikes on EcoG of the remaining cortex and clinical outcome with respect to seizure control.54 Falconer and colleagues6 also recorded EcoG during surgery but performed a standard temporal lobectomy on all patients regardless of the electrical findings. The standard resection included a 6 to 7 cm lateral removal (anterior-posterior) as measured from the temporal pole and 2 to 3 cm of the mesial structures, including the amygdala and the anterior hippocampus. This standard lobectomy produced good outcomes and was adopted by most centers. Large lateral temporal resections cause visual field cuts, typically an upper and outer quadrantanopsia. In the dominant hemisphere, language deficits, especially word–finding difficulties, anomias, and verbal memory impairment, are common.20,21,39
Several modifications of the standard lobectomy were developed to avoid or limit the extent of these common postoperative deficits. Larger resections were associated with better seizure control. Some surgeons, however, attributed this outcome to the extent of the mesial resection. Spencer et al.57 modified the standard lobectomy to limit the lateral resection but mesial resection of the hippocampus was aggressive. The Spencer modification spares the entire superior temporal gyrus, and only the anterior 3 to 4 cm of the middle and inferior temporal gyri are resected. The temporal-occipital fasciculus is followed posteriorly, facilitating complete removal of the hippocampus and yielding a 4-cm mesial resection. This modification gained widespread use because seizure control improved and postoperative impairments were reduced.
The optimal extent of mesial resection has been debated for many years.9,10,12-15,17,19,26-28,35,36,41,47-49,55,56,60-64,66 The improved outcomes of patients who underwent complete hippocampectomies supported the role of aggressive mesial resections. Some clinicians feared that memory impairment would worsen with complete removal of the hippocampus. Wyler et al.64 reported a prospective randomized study aimed at resolving the question of the extent of mesial resection. All patients underwent a standard 4-cm lateral resection. One cohort underwent a partial 2.5-cm hippocampectomy, and the other cohort underwent a complete or 4-cm mesial resection. All patients were followed for seizure control and neuropsychiatric testing. The seizure-free rate was dramatically higher in patients who had undergone a complete mesial resection (69%) than in those who had undergone a partial resection (38%). Furthermore, memory or language deficits did not increase in patients with complete hippocampal resections. In fact, the most important indicator for stable or improved postoperative memory and cognitive function was the attainment of a seizure-free status, which favored complete mesial resection.
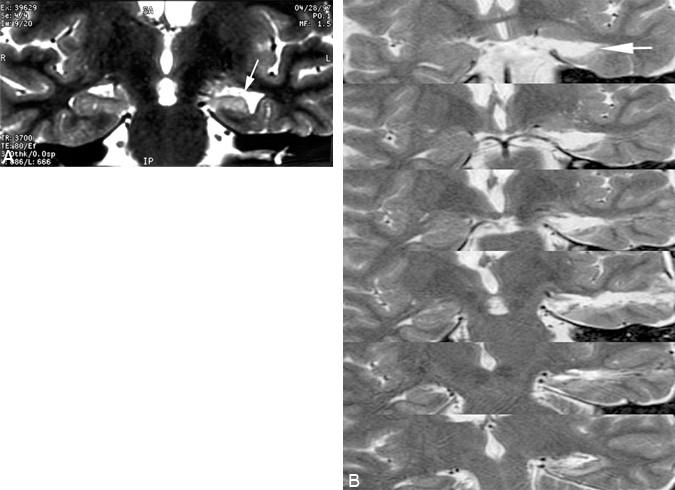
Selective Amygdalohippocampectomy
MTS is the most common pathological finding in the resected specimens of patients who have undergone surgery for temporal lobe epilepsy (Fig. 1A).15,26,40,49 Interestingly, the lateral temporal cortex in such resected specimens is routinely normal. A patient may have cortical epilepsy in the temporal lobe similar to frontal lobe epilepsy, but the condition is rare compared to MTS. Temporal lobe epilepsy has multiple causes: childhood encephalitis or infantile febrile seizures, trauma causing contusions and scarring, hypoxic brain injury, and lesions such as vascular malformations, hamartomas, or tumors (e.g., gangliogliomas or various astrocytomas). MTS may be associated with or caused by any of these conditions and is considered a final common pathway for the relative propensity of the temporal lobe to develop chronic epilepsy. The frequent histopathological observation of normal lateral cortex in resected specimens with MTS prompted the questions of whether any lateral cortex needed to be resected 40,49 or whether the removal of the mesial temporal lobe structures involved in the MTS would be sufficient to cure seizures in appropriate patients.
Niemeyer 37,38 reported a small group of patients with temporal lobe epilepsy who underwent resection of the amygdala and anterior 3 cm of the hippocampus by means of a transventricular approach through the middle temporal gyrus. The procedure was abandoned because the operation, which was performed before the development of the operating microscope, was technically difficult. The few patients who underwent this procedure did well with respect to seizure control and cognitive function.
Yasargil,65,66 who is credited with popularizing the operating microscope and microneurosurgery, developed the transsylvian approach for selective amygdalohippocampectomy in the 1970s. In terms of seizure control, the patients in his original series did as well as patients who had undergone large temporal resections. His patients later underwent neuropsychiatric testing and were found to have favorable cognitive outcomes. Other surgeons adopted this approach, but it too failed to gain widespread popularity because it was technically difficult and was associated with an increased risk of injury to the vascular structures of the Sylvian fissure.11,24,28,50,52,59 In this approach, the anterior temporal lobe is disconnected from the rest of the brain because the white matter temporal stem is transected to gain access to the ventricle.
In 1993 Hori et al.23 described a new subtemporal approach for selective amygdalohippocampectomy. The surgeon is positioned below the patient and faces cephalad. A temporal craniotomy is performed, and the lateral lobe is retracted upward to allow resection of the mesial lobe structures. In this initial report of four patients, two patients had transient diplopia and two had persistent seizures after the procedure. All had excellent neuropsychiatric outcomes. Unfortunately, no postoperative imaging was shown to document the extent of resection.
In 1996, Park et al.42 reported eight patients who underwent a large temporal craniotomy and a similar subtemporal transparahippocampal approach. To decrease the extent of temporal lobe retraction, the authors recommended hyperventilation, lumbar cisternal drainage, and the administration of mannitol. Seven of their eight patients were rendered seizure free. Postoperatively, however, one patient developed two complications, including a visual field defect, and another developed a significant memory impairment. Again, no postoperative imaging studies were published with the series and the extent of hippocampal resection was not discussed. Long-term follow-up for seizure control is not yet available.
At the 1997 World Stereotactic and Functional Neurosurgery Annual Conference, Olivier (personal communication) reported 139 patients who underwent selective amygdalohippocampectomy at the Montreal Neurological Institute. A lateral approach through the middle temporal gyrus was used. The seizure control rates reported were excellent—85% of the patients were free of seizures after 1 year. Again, however, no neuropsychiatric follow-up was available. Nonetheless, outcomes appeared favorable compared to patients who had undergone temporal resections.
Minimal Access, Wand-Guided Selective Amygdalohippocampectomy
The advent of frameless stereotactic guidance with technology such as the ISG Wand16 has permitted the development of a minimal access technique to perform selective amygdalohippocampectomy procedures in appropriately selected patients. The viewing wand is registered to a set of MR images of the patient obtained the morning of surgery. A linear incision is planned centered over the most appropriate sulcus for gaining access to the temporal horn of the lateral ventricle and hippocampus. Gyral-sulcal anatomy is quite variable, and the optimal sulcus used for entry must be individually selected, with the most inferior sulcus being preferred.
A small craniotomy is centered over the selected sulcus. The stereotactic guidance probe is used to assure that the trajectory to the temporal horn at the level of the pes hippocampus is correct. When the ventricle is opened, the ependymal surface and choroid plexus are visible as well as the unmistakable anatomical landmarks of the hippocampus and choroidal fissure. We have performed this minimally invasive selective amygdalohippocampectomy on more than 30 patients (Fig. 1B). More than 90% of patients have been rendered free or almost free of seizures as a result of the procedure.
A pending study will compare preoperative and postoperative cognitive function, as measured by neuropsychiatric testing, in patients who undergo selective amygdalohippocampectomy with patients who undergo standard temporal lobectomy. Although the data are not yet available, the clear impression of the treating staff is that the cognitive function of patients treated with selective amygdalohippocampectomy is significantly better than in the other group. Consequently, selective amygdalohippocampectomy through the minimal access approach described above has become our procedure of choice for all patients with refractory temporal lobe epilepsy preoperatively diagnosed with MTS.
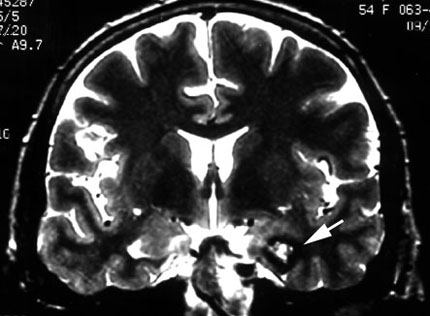
Cortical Resection Guided by EcoG
Nontemporal lobe epilepsy is usually associated with a structural lesion but can be nonlesional.40 Many benign or malignant tumors cause epilepsy, and the heralding symptom often is a seizure event. Vascular lesions, such as arteriovenous malformations and cavernous malformations, also often cause seizures (Fig. 2).12,22,59 In fact, seizures are the most common presenting symptom of cavernous malformations. The type of seizure is directly related to the location of the lesion.
The fundamental concept to consider with lesional epilepsy is that lesions themselves are not the source of the seizures. Rather, the brain tissue adjacent to the lesion becomes irritated or altered in some way that induces seizures. Other than heterotopias or neuronal tumors, the lesions are not composed of neuronal cells and therefore cannot produce seizures. Occasionally, seizures arise from regions of the brain distant from a visible structural lesion.
Consequently, the management of patients with lesional epilepsy has long been a dilemma. Is lesionectomy alone sufficient to cure the seizures, or is extralesional cortical resection required? If additional cortical resection is necessary, then how extensive must the resection be and at what risk to the patient for loss of functional capacity?
The answers to the first two questions are both yes. Complete removal of a lesion such as a benign tumor or vascular malformation can and often does completely cure the epilepsy.12,22,59 Alternatively, however, many patients have been documented to need significant extralesional cortical resections to control their seizures. In nonlesional cases, significant cortical resection is required to remove the epileptogenic zone, but the extent of the resection must be defined. Furthermore, vital regions or eloquent areas of the brain need to be delineated accurately and preserved if nearby regions must be resected.
One way to determine the extent of cortical resection while avoiding vital functional regions is by EcoG and functional brain mapping. The implantation of subdural electrodes, video-EcoG-telemetry, and functional brain mapping allow surgeons to plan the extent of resection required while sparing vital areas. This technique has been highly successful.40
Occasionally, eloquent areas are directly involved in epileptogenesis. In such cases, the technique of multiple subpial transections, as described by Morrell et al.,33 can be used with moderate success. The technique involves transection of horizontal fibers in the cortex but preserves vertical fibers. Preservation of function is excellent—usually there are no perceptible differences. However, seizure control can be less than that achieved by resection.
Hemispherectomy, first described by Krynaw in 1950,29 remains one of the most successful treatments for intractable seizures in appropriately selected patients. Typically, patients are children with a congenitally malformed hemisphere and unilateral seizure onset originating from the nonfunctioning side of the brain. Complete elimination of all seizures with no change in neurological function can be achieved in more than 80% of the patients. The late complication of hemosiderosis after a complete anatomical hemispherectomy prompted the development of many modifications of the procedure, termed modified or functional hemispherectomy or hemispheric deafferentation.
We have recently performed two modified functional hemispherectomies through minimal access craniotomies using frameless stereotactic neuronavigational guidance and have obtained excellent initial results. Long-term follow-up is pending, but the advantages of a small incision, minimal blood loss, and complete disconnection of the hemisphere without removal of tissue appear to be optimal.
Corpus callosotomy must be mentioned for the sake of completeness. This procedure can be useful for patients with drop attacks resulting from bilateral seizures like those associated with the Lennox-Gastaut syndrome. The procedure is palliative for drop attacks only and seldom renders patients free of seizures. Again, newer techniques with neuronavigation allow the procedure to be performed with greater safety and smaller incisions.
In some European centers, Gamma Knife radiosurgery has been advocated as an alternative means of treating lesional epilepsy and also as an alternative means of performing corpus callosotomies and selective amygdalohippocampectomies. A complete discussion of all the controversies are beyond the scope of this article. However, it must be considered experimental at this time.
Conclusion
Advances in diagnostic modalities that permit epileptogenic zones to be localized and the development of new surgical devices such as frameless stereotactic navigation have facilitated the development of less invasive surgical approaches that are more successful than past techniques. All patients with medically intractable epilepsy should be referred to a specialized center for complete evaluation. Most referred patients can be rendered free of seizures or nearly so after undergoing a complete evaluation and contemporary neurosurgical intervention as outlined above. Although some patients will not be candidates for surgical intervention, many can benefit from such an evaluation and medical control of their seizures may improve if they are treated with the new anticonvulsant medications.
References
- Behrens E, Schramm J, Zenter J, et al: Surgical and neurological complications in a series of 708 epilepsy surgery procedures. Neurosurgery 41:1-10, 1997
- Birri R, Perret E, Wieser HG: Der Einflub verschiedener Temporallappenoperationen auf das Gedächtnis bei Epileptikern. Nervenarzt 53:144-149, 1982
- Burchiel KJ, Clarke H, Ojemann GA, et al: Use of stimulation mapping and corticography in the excision of arteriovenous malformations in sensorimotor and language-related neocortex. Neurosurgery 24:322-327, 1989
- Engel JJ: Surgery for seizures. N Engl J Med 334:647-652, 1996
- Falconer MA: Discussion on the surgery of temporal lobe epilepsy: Surgical and pathological aspects. Proc R Soc Med 46:971, 1953
- Falconer MA, Hill D, Meyer A, et al: Treatment of temporal lobe epilepsy by temporal lobectomy: A survey of findings and results. Lancet 1:827-835, 1955
- Feindel W: Development of surgical therapy of epilepsy at the Montreal Neurological Institute. Can J Neurol Sci 18:549-553, 1991
- Feindel W, Penfield W: Localization of discharge in temporal lobe automatism. Arch Neurol Psychiatry 72:605-630, 1954
- Feindel W, Rasmussen T: Temporal lobectomy with amygdalectomy and minimal hippocampal resection: Review of 100 cases. Can J Neurol Sci 18:603-605, 1991
- Fried I: Anatomic temporal lobe resections for temporal lobe epilepsy. Neurosurg Clin N Am 4:233-242, 1993
- Fujii K, Lenkey C, Rhoton ALJ: Microsurgical anatomy of the choroidal arteries: Lateral and third ventricles. J Neurosurg 52:165-188, 1980
- Goldring S, Edwards I, Harding GW, et al: Results of anterior temporal lobectomy that spares the anygdala in patients with complex partial seizures. J Neurosurg 77:185-193, 1992
- Goldstein LH, Polkey CE: Behavioural memory after temporal lobectomy or amygdalo-hippocampectomy. Br J Clin Psychol 31:75-81, 1992
- Goldstein LH, Polkey CE: Everyday memory after unilateral temporal lobectomy or amygdalo-hippocampectomy. Cortex 28:189-201, 1992
- Goldstein LH, Polkey CE: Short-term cognitive changes after unilateral temporal lobectomy or unilateral amygdalo-hippocampectomy for the relief of temporal lobe epilepsy. J Neurol Neurosurg Psychiatry 56:135-140, 1993
- Golfinos JG, Fitzpatrick BC, Smith LR, et al: Clinical use of a frameless stereotactic arm: Results of 325 cases. J Neurosurg 83:197-205, 1995
- Gonçalves-Ferreira A, Miguéns J, Farias JP, et al: Selective amygdalohippocampectomy: Which route is the best? Stereotact Funct Neurosurg 63:182-191, 1994
- Gonser A, Perret E, Wieser HG: Ist der hippokampus für Lern-und Gedächtnisprozesse notwendig? Nervenarzt 57:269-275, 1986
- Helmstaedter C, Elger CE, Hufnagel A, et al: Different effects of left anterior temporal lobectomy, selective amygdalohippocampectomy, and temporal cortical lesionectomy on verbal learning, memory, and recognition. J Epilepsy 9:39-45, 1996
- Hermann BP, Wyler AR, Somes G: Language function following anterior temporal lobectomy. J Neurosurg 74:560-566, 1991
- Hermann BP, Wyler AR, Somes G, et al: Dysnomia after left anterior temporal lobectomy without functional mapping: Frequency and correlates. Neurosurgery 35:52-57, 1994
- Heros RC: Arteriovenous malformations of the medial temporal lobe. J Neurosurg 56:44-52, 1982
- Hori T, Tabuchi S, Kurosaki M, et al: Subtemporal amygdalohippocampectomy for treating medically intractable temporal lobe epilepsy. Neurosurgery 33:50-57, 1993
- Huther G, Dörfl J, Van der Loos H, et al: Microanatomic and vascular aspects of the temporomesial region. Neurosurgery 43:1118-1136, 1998
- Kelly PJ, Sharbrough FW, Kall BA, et al: Magnetic resonance imaging-based computer-assisted stereotactic resection of the hippocampus and amygdala in patients with temporal lobe epilepsy. Mayo Clin Proc 62:103-108, 1987
- Kitchen ND, Thomas DGT, Thompson PJ, et al: Open stereotactic amygdalohippocampectomy—clinical, psychometric, and MRI follow-up. Acta Neurochir (Wien) 123:33-38, 1993
- Kondo S, Takenobu A, Tabuchi S, et al: Subtemporal amygdalohippocampectomy for medically intractable temporal lobe epilepsy. Jpn J Psychiatry Neurol 47:273-274, 1993
- Kratimenos GP, Pell MF, Thomas DGT, et al: Open stereotactic selective amygdalo-hippocampectomy for drug resistant epilepsy. Acta Neurochir (Wien) 116:150-154, 1992
- Krynauw RA: Infantile hemiplegia treated by removing one cerebral hemisphere. J Neurol Neurosurg Psychiatry 13:243-267, 1950
- Kuhl DE, Engel J, Jr., Phelps ME, et al: Epileptic patterns of local cerebral metabolism and perfusion in humans determined by emission computed tomography of 18FDG and 13NH3. Ann Neurol 8:348-360, 1980
- Leonard G: Temporal lobe surgery for epilepsy: Neuropsychological variables related to surgical outcome. Can J Neurol Sci 18:593-597, 1991
- Marks DA, Katz A, Hoffer P, et al: Localization of extratemporal epileptic foci during ictal single photon emission computed tomography. Ann Neurol 31:250-255, 1992
- Morrell F, Whisler WW, Bleck TP: Multiple subpial transection: A new approach to the surgical treatment of focal epilepsy. J Neurosurg 70:231-239, 1989
- Nakasato N, Levesque MF, Babb TL: Seizure outcome following standard temporal lobectomy: Correlation with hippocampal neuron loss and extrahippocampal pathology. J Neurosurg 77:194-200, 1992
- Nayel MH, Awad IA, Luders H: Extent of mesiobasal resection determines outcome after temporal lobectomy for intractable complex partial seizures. Neurosurgery 29:55-61, 1991
- Naylor AS, Rogvi-Hansen B, Kessing L, et al: Psychiatric morbidity after surgery for epilepsy: Short term follow up of patients undergoing amygdalohippocampectomy. J Neurol Neurosurg Psychiatry 57:1375-1381, 1994
- Niemeyer P: The transventricular amygdalohippocampectomy, in Baldwin M, Bailey P (eds): Temporal Lobe Epilepsy. Springfield, IL: Charles C Thomas, 1958, pp 461-482
- Niemeyer P: Amygdalohippocampectomy for temporal lobe epilepsy: Microsurgical technique (abstract). Excepta Medica 293:20, 1973
- Ojemann GA, Dodrill CB: Verbal memory deficits after left temporal lobectomy for epilepsy. J Neurosurg 62:101-107, 1985
- Olivier A: Commentary: Cortical resections, in Engel JJ (ed): Surgical Treatment of the Epilepsies. New York: Raven, 1987, pp 405-416
- Park TS: Amygdalohippocampectomy (Response to letter to editor by Rapport RL). J Neurosurg 86:1072, 1997
- Park TS, Bougeois BF, Silbergeld DL, et al: Subtemporal transparahippocampal amygdalohippocampectomy for surgical treatment of mesial temporal lobe epilepsy. J Neurosurg 85:1172-1176, 1996
- Penfield W: The radical treatment of traumatic epilepsy and its rationale. J Can Med Assoc 23:183-197, 1930
- Penfield W, Baldwin M: Temporal lobe seizures and the technic of subtotal temporal lobectomy. Ann Surg 136:625-634, 1952
- Penfield W, Boldrey E: Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60:398-443, 1937
- Penfield W, Flanigin H: Surgical therapy of temporal lobe seizures. Arch Neurol Psychiatr 64:491-500, 1950
- Phillips J, Fouad M: Comparative study between anterior temporal lobectomy and selective amygdalohippocampectomy in the management of intractable temporal lobe seizures. Br J Neurosurg 11:476-477, 1997
- Rasmussen T, Feindel W: Temporal lobectomy: Review of 100 cases with major hippocampectomy. Can J Neurol Sci 18:601-602, 1991
- Renowden SA, Matkovic Z, Adams CBT, et al: Selective amygdalohippocampectomy for hippocampal sclerosis: Postoperative MR appearance. AJNR 16:1855-1861, 1995
- Rhoton ALJ, Fujii K, Fradd B: Microsurgical anatomy of the anterior choroidal artery. Surg Neurol 12:171-187, 1979
- Saint-Hilaire JM, Richer F, Turmel A, et al: Relative localizing value of common tests used in the preoperative investigation of epileptic patients. Can J Neurol Sci 18:598-600, 1991
- Schaller C, Zenter J: Vasospastic reactions in response to the transsylvian approach. Surg Neurol 49:170-175, 1998
- Schulder M, Maldjian JA, Liu W-C, et al: Functional image-guided surgery of intracranial tumors located in or near the sensorimotor cortex. J Neurosurg 89:412-418, 1998
- Schwartz TH, Bazil CW, Walczak TS, et al: The predictive value of intraoperative electrocorticography in resections for limbic epilepsy associated with mesial temporal sclerosis. Neurosurgery 40:302-311, 1997
- Shimizu H, Suzuki I, Ishijima B: Zygomatic approach for resection of mesial temporal epileptic focus. Neurosurgery 25:798-801, 1989
- Siegel AM, Wieser HG, Wichmann W, et al: Relationships between MR-imaged total amount of tissue removed, resection scores of specific mediobasal limbic subcompartments and clinical outcome following selective amygdalohippocampectomy. Epilepsy Res 6:56-63, 1990
- Spencer DD, Spencer SS, Mattson RH, et al: Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery 15:667-671, 1984
- Spencer SS, Spencer DD, Williamson PD, et al: Combined depth and subdural electrode investigation in uncontrolled epilepsy. Neurology 40:74-79, 1990
- Vajkoczy P, Karakow K, Stodieck S, et al: Modified approach for the selective treatment of temporal lobe epilepsy: Transsylvian-transcisternal mesial en bloc resection. J Neurosurg 88:855-862, 1998
- Wieser HG: Selective amygdalo-hippocampectomy for temporal lobe epilepsy. Epilepsia 29:S100-S113, 1988
- Wieser HG: Selective amygdalohippocampectomy: Indication and follow-up. Can J Neurol Sci 18:617-627, 1991
- Wieser HG, Yasargil MG: Selective amygdalohippocampectomy as a surgical treatment of mesiobasal limbic epilepsy. Surg Neurol 17:445-457, 1982
- Wyler AR: Recent advances in epilepsy surgery: Temporal lobectomy and multiple subpial transections. Neurosurgery 41:1294-1302, 1997
- Wyler AR, Hermann BP, Somes G: Extent of medial temporal resection on outcome from anterior temporal lobectomy: A randomized prospective study. Neurosurgery 37:982-990, 1995
- Yasargil MG, von Ammon K, Cavazos E, et al: Tumours of the limbic and paralimbic systems. Acta Neurochir (Wien) 118:40-52, 1992
- Yasargil MG, Wieser HG, Valavanis A, et al: Surgery and results of selective anygdalahippocampectomy in one hundred patients with nonlesional limbic epilepsy. Neurosurg Clin N Am 4:243-261, 1993
