CT Perfusion Imaging Detects Cerebral Hypoperfusion Caused by Aneurysm Clip
Erin C. Prenger, DO
Joseph M. Zabramski, MD*
Division of Neuroradiology and *Division of Neurological Surgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
We present a case report in which computed tomography perfusion imaging was useful in documenting a reversible hypoperfusion in a patient with fluctuating neurologic deficits following clipping of a ruptured internal carotid artery aneurysm. Repositioning the clip resulted in restoration of symmetric blood flow on follow-up imaging and led to a good clinical outcome.
Key Words: cerebral blood flow, computed tomographic perfusion imaging
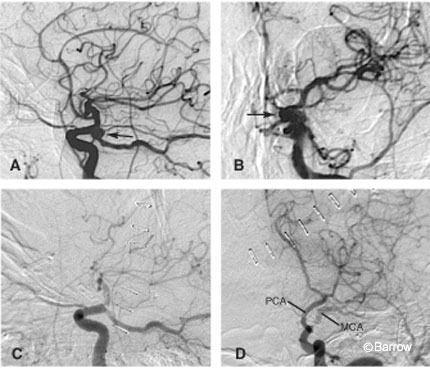
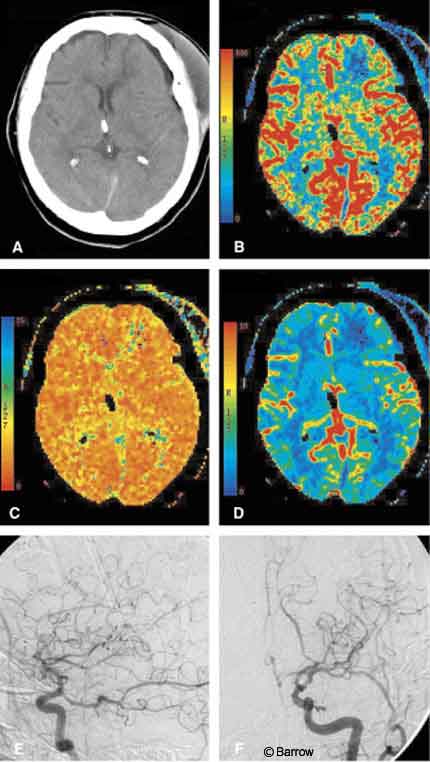
and (D) anteroposterior postoperative angiograms show obliteration of the aneurysm; however, there is marked narrowing of the supraclinoid right ICA secondary to clip placement. Note the marked delay in filling of the middle cerebral artery (MCA) territory. PCA, posterior cerebral artery.
Recent advances in computed tomography (CT) have resulted in the development of new techniques for evaluating cerebral blood flow (CBF), including CT perfusion imaging. CT perfusion imaging uses an intravenous bolus injection of contrast in conjunction with rapid, dynamic image acquisition to provide a quantitative assessment of CBF and the resulting brain perfusion. This technique quantitates CBF (ml/100 gm/min), cerebral blood volume (CBV, ml/100 gm), and mean transit time (MTT, sec). CT perfusion imaging is a useful adjunct for differentiating cerebral infarction from reversible ischemia and as a tool for evaluating a patient’s response to therapy.
Case Report
A 76-year-old, right-handed woman suffered from acute subarachnoid hemorrhage when an aneurysm on her left internal carotid artery (ICA) ruptured. The aneurysm was obliterated surgically with a fenestrated aneurysm clip that encircled the proximal ICA. Initially, she did well after surgery, but about 36 hours thereafter she developed a mild right hemiparesis associated with a decline in systemic blood pressure. The patient’s deficits rapidly cleared with the initiation of hypervolemia and mild hypertensive therapy. Cerebral angiography confirmed complete obliteration of the aneurysm; however, the clip also had significantly narrowed the ICA (Fig. 1).
CT performed the same day showed a relative decrease in CBF and prolonged MTT in the left hemisphere compared to the right. Evaluation of quantitative CBF revealed adequate blood flow values (>60 ml/100 gm/min) in the left hemisphere (Fig. 2). Although these findings suggested reduced flow through the left ICA, they confirmed adequate perfusion of the left hemisphere after the hypervolemic-hypertensive therapy.
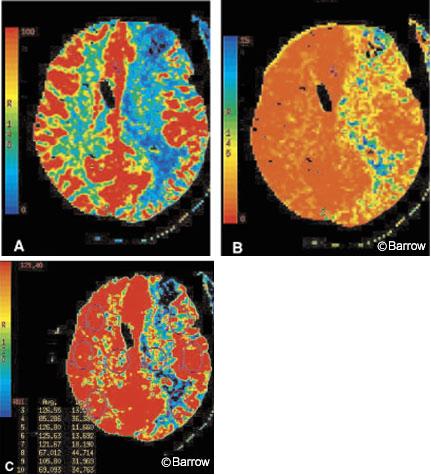
On the basis of the patient’s findings on angiography and CT perfusion imaging, a second surgery was performed to reposition the aneurysm clip. Postoperatively, the patient did well. CT perfusion imaging performed one day after the clip was repositioned showed that CBF and MTT in the left hemisphere had normalized (Fig. 3).
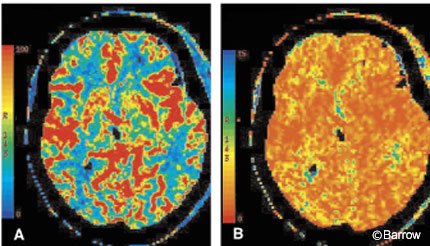
Follow-up CT, CT perfusion imaging, and cerebral angiography performed one week after the initial surgery confirmed normal and symmetric CBF, CBV, and MTT. A small, hypoperfused area of encephalomalacia was present in the left frontal region. The caliber of the ICA was normal (Fig. 4).
The patient remained neurologically stable and was transferred to neurorehabilitation on her 28th hospital day and subsequently returned home.
[one_half]
[/one_half]
[one_half_last]
[/one_half_last]
Discussion
Narrowing of the ICA, either from intrinsic atheromatous disease or as a potential complication of vascular surgery, places the ipsilateral hemisphere at risk for infarction.
Degree of stenosis, collateral circulation, and cerebral perfusion pressure determine the degree of cerebral ischemia and predict the potential risk of infarction. Although cerebral angiography can accurately define stenosis and collateralization, it cannot accurately reflect cerebral perfusion.
Newer functional imaging techniques, including CT perfusion imaging, provide a minimally invasive means of quantitating CBF and of defining areas of the brain at risk for infarction. In this case, CT perfusion imaging showed the asymmetric flow to the left hemisphere predicted by angiography.
More importantly, it confirmed the effectiveness of temporizing systemic measures to increase the patient’s cerebral perfusion pressure. The resolution of the stenosis resulted in normalization of cerebral perfusion without subsequent infarction, as confirmed by the follow-up CT and CT perfusion imaging.