
Microsurgical Anatomy of the Anterior Cerebral Circulation: Part 1. The Infraclinoid Internal Carotid Artery
Mauro A. T. Ferreira, MD
Iman Feiz-Erfan, MD
Pushpa Deshmukh, PhD
Joseph M. Zabramski, MD
Mark C. Preul, MD
Robert F. Spetzler, MD
Division of Neurological Surgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
The ability to operate on and preserve vessels of the brain depends on thorough knowledge of vascular microanatomy. This article reviews the microsurgical anatomy of the anterior circulation of the brain, focusing on the internal carotid artery (ICA) and its anatomical relationships as it travels from the neck toward the skull base. The relevant anatomical relationships of the ICA in the neck, petrous temporal bone, cavernous sinus, and anterior clinoid process are illustrated by meticulous anatomical dissections. The surgical implications of such knowledge are also highlighted. Special attention is paid to the cervical segment of the ICA, which is routinely exposed during carotid endarterectomy; to the petrous ICA, which may be exposed to obtain proximal vascular control or to place a vein graft bypass; and to the clinoid segment of the ICA, which may be exposed during surgery for proximal ICA aneurysms.
Key Words: carotid cave, cavernous sinus, clinoid segment of ICA, internal carotid artery, intracavernous carotid artery, microsurgical anatomy, petrous carotid artery
Knowledge of the direction of blood vessels, their typical anatomy and variations, and the location of perforating branches and their collateral network is fundamental to treating vascular lesions of the brain. The importance of detailed knowledge of microsurgical anatomy is crucial given the exquisite anatomical information provided by contemporary neuroimaging modalities and the ability of neurosurgeons to preserve small and delicate, yet eloquent, anatomical structures. The anterior circulation of the brain is defined by the internal carotid artery (ICA) system. It includes the ICA and its branches, the collateral vessels to the contralateral side, and the collateral vessels to the posterior circulation.
The Internal Carotid Artery
The appropriate classification of the segments of the ICA is still a matter of debate, and the nomenclature applied to the various segments of the ICA has been confusing. In 1938 Fischer[5] first described the segments of the ICA based on angiographic observations. He divided the ICA into five segments, from C1 to C5. However, the various segments were named opposite the direction of blood flow. Nor did this classification address the specific anatomical location of the different segments or their clinical and surgical implications. It also excluded the extracranial ICA.
In 1981 Gibo et al.[6] described the segments of the ICA in a numerical scale from C1 to C4. This system was based on careful anatomical observations, and the segments of the supraclinoid ICA were elegantly described and subdivided. The classification followed the direction of blood flow and included the entire length of the artery from the cervical region to the intracranial arterial bifurcation. However, it did not describe the particular anatomic features of the clinoid segment.
In 1996 Bouthillier et al.[1] developed an accurate and useful classification scheme that described the segments of the ICA according to the direction of blood flow and the particular anatomical features of each segment. They also discussed the application of the system to the current understanding of microsurgical anatomy and its surgical implications. They included the clinoid segment of the ICA and added another segment located between the petrous and intracavernous ICA segments. Because this segment was related to the foramen lacerum, it was referred to as the lacerum segment.
We recognize and describe five segments of the ICA: the cervical, the petrous, the intracavernous, the clinoid, and the supraclinoid segments. The supraclinoid portion of the ICA is further subdivided into three segments: the ophthalmic, the communicating, and the choroidal segments in accordance to the classification provided by Gibo et al.[6] The microsurgical anatomy of the cervical, petrous, cavernous, and clinoid segments of the ICA are described in this article. A future article will be devoted to the supraclinoid segment.
[one_third]
[/one_third]
[one_third]
[/one_third]
[one_third_last]
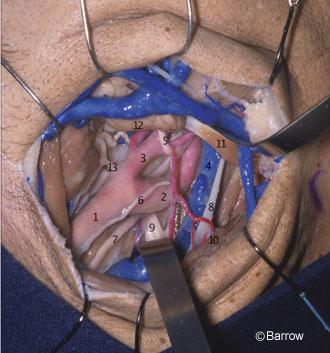
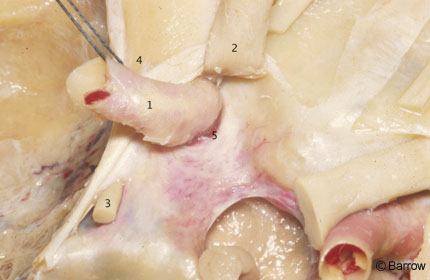
Figure 3. Anatomy of the carotid bifurcation at the anterior triangle of the neck as seen during carotid endarterectomy (left side). An oblique incision through the anterolateral aspect of the neck exposes the carotid bifurcation. The dissection proceeds medially to the internal jugular vein (IJV). The posterior belly of the digastric muscle crosses the superior aspect of the exposure. The ICA ascends toward the carotid canal medial to the styloid process. 1=common carotid artery, 2=ICA, 3=external carotid artery, 4=IJV, 5=hypoglossal nerve, 6=ansa cervicalis, 7=vagus nerve, 8=accessory nerve, 9=cervical plexus, 10=sternocleidomastoid muscle, 11=posterior belly of digastric muscle, 12=submandibular gland, and 13=hypoplastic superior thyroid artery.
[/one_third_last]
The Cervical Segment
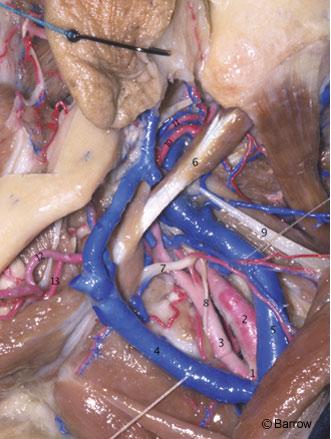
The ICA begins at the bifurcation of the common carotid artery at the level of the upper border of the carotid triangle of the neck. Initially superficial when medial to the sternocleidomastoid muscle and the internal jugular vein, the ICA assumes a much deeper position after passing medial to the posterior belly of the digastric muscle.[22] Below the digastric muscle at the level of the common carotid artery bifurcation, the ICA is crossed by the hypoglossal nerve, the ansa cervicalis, and the lingual and facial veins (Fig. 1). Medial to the digastric muscle, the ICA is crossed by the stylohyoid muscle and the occipital and posterior auricular arteries. Superior to the digastric muscle, the ICA is separated from the external carotid artery by the styloid process and the muscles attached to it. The styloid process is a bony spicule projecting from the base of the tympanic bone into the infratemporal fossa and is the site of attachment of the styloglossus, stylopharyngeus, and stylohyoid muscles. It is located immediately anterior to the emergence of the facial nerve from the temporal bone at the stylomastoid foramen (Fig. 2).[2,9,22] When the styloid process is resected and the muscles attached to it are reflected downward, the ICA is revealed as it enters the carotid canal medial to the tympanic bone.[2,9] On its ascending course toward the base of the skull, the ICA lies medial to the vagus nerve and internal jugular vein.[2] Although questioned by some,[1] the ICA is surrounded by a dense sheath of connective tissue at its entrance into the carotid canal and is separated from the internal jugular vein by the hypoglossal nerve and the nerves exiting from the jugular foramen.[2,9] The bifurcation of the ICA is routinely exposed in the neck for carotid endarterectomy (Figs. 3 and 4).
[one_half]
[/one_half]
[one_half_last]
[/one_half_last]
The Petrous Segment
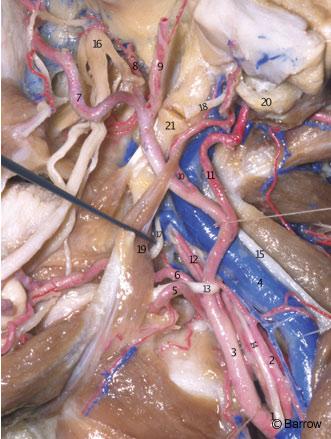
The ICA enters the cranial cavity by traversing the periosteal-lined carotid canal located in the petrous portion of the temporal bone in front of and intimately related to the jugular foramen and internal jugular vein (Fig. 5).[9] The connective tissue that surrounds the artery inside this bony canal is thick and adherent to the artery at its entrance. It makes mobilization of the artery difficult at this point.[2] The relationships of the petrous segment of the ICA to adjacent structures include the facial canal, the internal acoustic meatus, the cochlea, the geniculate ganglion, the facial nerve, the greater and lesser petrosal nerves, the trigeminal nerve, the middle ear, the eustachian tube, the middle meningeal artery, and the tensor tympani muscle.[15] If unprotected, these anatomical structures can be injured during surgical exposure of the petrous ICA (Figs. 6-9).
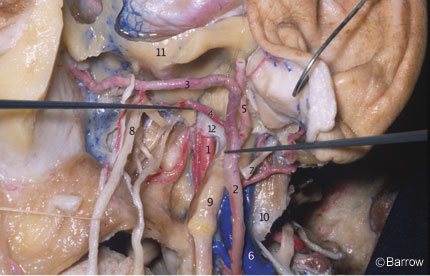
The petrous ICA is divided into two segments that join at the genu: a vertical or ascending segment and a horizontal segment. As measured by Paullus et al.,[15] the vertical segment has a mean length of 10.5 mm (range, 6.0 to 15 mm). It is directed superiorly before turning anteromedially at the genu to form the horizontal segment. In relationshipto the vertical segment are the jugular fossa posteriorly, the eustachian tube anteriorly, and the tympanic bone anterolaterally (Fig. 6).
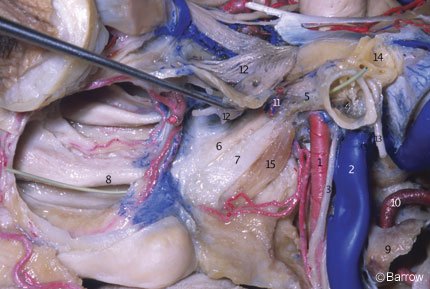
The horizontal segment has a mean length of 20.1 mm (range, 15 to 25.1 mm) and begins at the genu (Fig. 7); it passes anteriorly and medially, anterior to the cochlea. The cochlea is usually posterior or posterosuperior to the genu; the distance from the genu to the basal turn of the cochlea averages 2.1 mm (range, 0.6 to 10.0 mm). The cochlea can be damaged during exposure of the ICA in the petrous bone (Figs. 8 and 9). The medial part of the roof of the horizontal portion of the canal is formed by dura or a thin plate of bone that separates the carotid artery from the Gasserian ganglion.
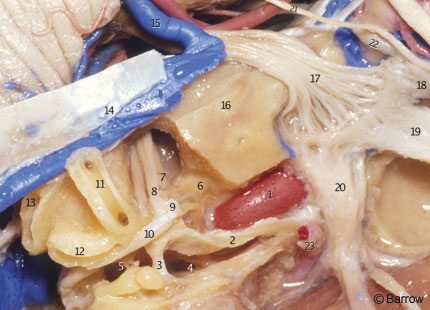
The eustachian tube and the tensor tympani muscle are located anterior and parallel to the horizontal segment, below the floor of the middle fossa, and are separated from it by a bony septum of variable size (Fig. 9). The entrance of the eustachian tube into the middle ear cavity lies directly anterior and superior to the genu of the carotid artery (Fig. 7).[15] The greater superficial petrosal nerve (GSPN) originates from the geniculate ganglion of the facial nerve and runs anteromedially under the dura of the middle fossa, toward the inferior surface of the trigeminal ganglion, and then to the pterygoid canal. The GSPN usually courses above and parallel to the horizontal portion of the carotid artery (Fig. 7). The GSPN runs in the groove for the GSPN and is usually attached to the dura overlying the middle fossa floor. Bone may be absent over the geniculate ganglion.[18] The middle ear cavity is located posterolateral to the genu and vertical portion of the carotid canal (Fig. 7). The junction of the facial canal and acoustic meatus lies an average of 2.3 mm (range, 2.0 to 2.8 mm) below the surface of the middle fossa. It can be exposed by locating the geniculate ganglion and following the facial nerve medially toward the meatus (Fig. 9).[15] The initial portion of the facial canal lies a mean of 6.6 mm (range, 4.0 to 10.0 mm) from the genu of the carotid canal. Because of this wide separation and the presence of the cochlea with its capsule located between the carotid genu and the facial canal, it is unusual for the facial nerve to be damaged at this point during surgical exposure of the carotid artery. However, care should be taken when exposing the middle fossa floor to avoid placing traction on the GSPN and geniculate ganglion. Such traction can cause postoperative facial nerve palsy, and the GSPN should be sharply cut during surgery.
The trigeminal ganglion makes an impression on the apex of the petrous bone and lies over the medial portion of the intrapetrous carotid artery (Figs. 7-9). The ganglion is separated from the vessel by dura or a variable layer of bone.[2] The length of the horizontal segment that can be exposed lateral to the trigeminal nerve averages 8.9 mm (range, 6.0 to 12.0 mm). This exposure can be improved if the mandibular branch of the trigeminal nerve is retracted or divided. This maneuver increases the mean length exposed to 20.1 mm (range, 17.5 to 28 mm).[2] Maximizing this exposure is particularly important during surgical procedures directed through the apex of the petrous bone. Division of the mandibular branch can be used to widen the length of the area exposed to perform a vascular anastomosis, to obtain proximal control of arterial bleeding, and to allow mobilization of the vertical and horizontal segments of the artery from the petrous bone.
The middle meningeal artery enters the cranial cavity through the foramen spinosum of the sphenoid bone. The foramen spinosum is medial and anterior to the geniculate ganglion (Figs. 7-9). This relationshipis important when performing extradural approaches to the floor of the middle fossa, either posteriorly or anteriorly. The arcuate eminence is another important landmark in the floor of the middle fossa. The foramen spinosum is located a mean of 4.7 mm (range, 2.5 to 8.0 mm) anterolateral to the carotid canal, measured along the posterior border of the third division of the trigeminal nerve.[15]
Inside the petrous bone, the ICA is surrounded by a venous plexus of variable size, an extension of the cavernous sinus within the periosteal covering of the distal part of the canal, and a network of sympathetic nerve fibers from the internal carotid branch of the sympathetic trunk. The internal carotid branch of the cervical sympathetic ganglia divides into two segments near the genu. A posterior branch sends some rootlets that accompany the cerebral arteries and the trochlear and trigeminal nerves. An anterior branch gives rise to the deeppetrosal nerve and sends some filaments to the abducent nerve and to the first division of the trigeminal nerve (Fig. 7).[2] The intrapetrous carotid artery has two branches. The caroticotympanic branch enters the tympanic cavity through a foramen in the wall of the vertical segment of the carotid canal. The vidian or pterygoid branch usually arises from the internal maxillary artery but may arise from the horizontal segment of the ICA. Often inconsistent, these branches are difficult to demonstrate in cadaveric preparations.[2] The arterial rami of the intrapetrous carotid artery were found in 23% of angiographic studies and in 38% of anatomic dissections.[17] Most of these branches were periosteal branches and the vidian artery. The frequency of the caroticotympanic artery is so low that its presence approaches being anomalous.
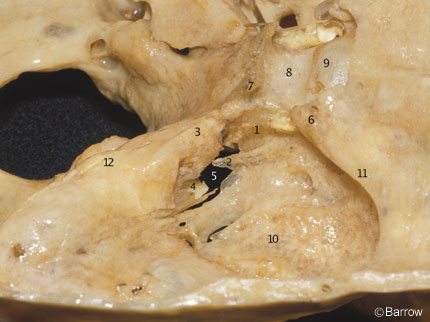
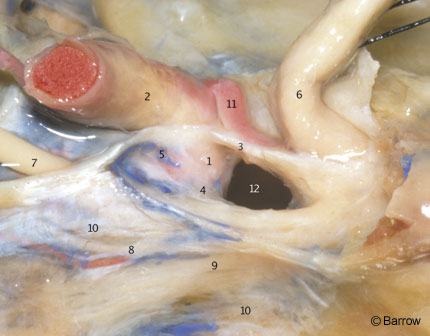
At the distal portion of the horizontal segment, the ICA runs superiorly and medially over the fibrocartilaginous tissue that fills the foramen lacerum. The foramen lacerum is located between the posterolateral body of the sphenoid and the apex of the petrous bone (Fig. 10). The transition between the petrous and the cavernous ICA occurs at the level of the petrolingual ligament (Fig. 11). This band of connective tissue extends from the lingula, a bony prominence located at the posteroinferior margin of the carotid sulcus to the petrous apex (Fig. 10). This ligament extends over the ICA just proximal to the point where the ICA assumes a vertical position on the posterolateral aspect of the body of the sphenoid bone to become intracavernous (Fig. 11).
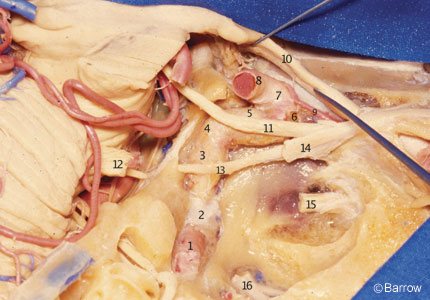
The Cavernous Segment
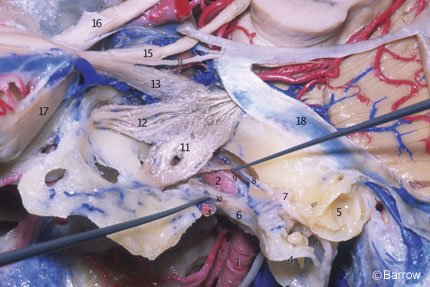
The cavernous portion of the ICA begins as the petrous ICA ascends from the foramen lacerum toward the posterior clinoid process. The initial ascending portion of the cavernous ICA is called the posterior vertical segment. This segment ends at the site where the artery turns anteriorly, forming a curve called the posterior bend, to assume a horizontal course inside the cavernous sinus. The horizontal portion passes anteriorly toward the anterior portion of the sinus, where it makes an upward curve called the anterior bend (Fig. 11). Distal to the anterior bend, the artery continues superiorly to form the anterior vertical segment, passing medial to the anterior clinoid process to pierce the roof of the cavernous sinus. The portion of the anterior vertical segment medial to the anterior clinoid process is the clinoid segment.[2] The horizontal segment of the ICA inside the cavernous sinus may be surgically accessed through Parkinson’s triangle, a triangular area in the lateral wall of the cavernous sinus limited superiorly by the trochlear nerve, inferiorly by the ophthalmic division of the trigeminal nerve, and posteriorly by the dura of the tentorium.
The most consistent arterial branches of the cavernous ICA are the meningohypophyseal trunk, the largest of the cavernous branches; the artery of the inferior cavernous sinus; and the capsular arteries of McConnell. Less frequently, the ophthalmic artery can arise within the cavernous sinus and pass through the superior orbital fissure to reach the orbit. Persistent trigeminal arteries can also originate from the posterior vertical segment of the cavernous ICA and pass posteriorly through the posterior wall of the sinus to join the basilar artery between the origin of the superior and anterior inferior cerebellar arteries. The microsurgical anatomy and the clinical relevance of the persistent trigeminal artery have been described by Suttner et al.[21]
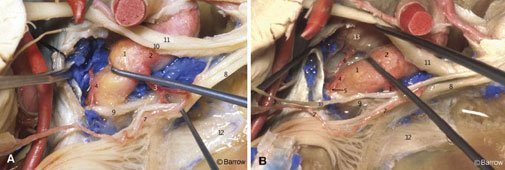
The meningohypophyseal trunk usually arises from the posterior aspect of the central third of the posterior bend of the artery at the level of the dorsum sellae and frequently gives rise to three branches: the tentorial artery, the dorsal meningeal artery, and the inferior hypophyseal artery. Separate origins can also be found for all three branches (Fig. 12).
The tentorial artery, or the artery of Bernasconi and Cassinari, is the most constant branch of the meningohypophyseal trunk. It passes posterolaterally to the roof of the cavernous sinus and along the free edge of the tentorium, giving rise to branches to the dura of the tentorium and dorsum sellae and to the third and fourth cranial nerves. When the tentorial artery is absent, a branch of the artery of the inferior cavernous sinus, the so-called marginal tentorial artery, can be found coursing posteriorly along the tentorial edge (Fig. 12). The dorsal meningeal artery arises from the meningohypophyseal trunk and passes posteriorly, underneath the petroclinoid (Gruber’s) ligament, to supply the dura of the upper clivus and dorsum sellae. The dorsal meningeal artery then anastomoses with its contralateral mate.
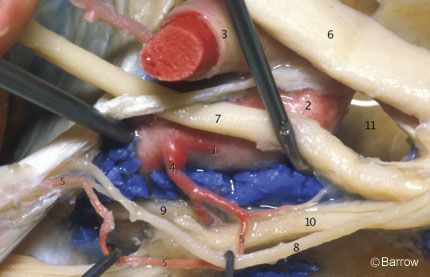
The inferior hypophyseal artery passes medially to the posterior pituitary capsule and lobe and anastomoses with its counterpart from the other side after supplying the dura of the sellar floor (Fig. 12). The arterial supply of the anterior lobe of the pituitary is provided by the superior hypophyseal arteries, branches of the ophthalmic segment of the supraclinoid portion of the ICA. The artery of the inferior cavernous sinus usually arises from the middle third of the inferior or lateral surfaces of the horizontal segment of the cavernous ICA (Fig. 13). It runs laterally, usually over the abducent nerve, and inferiorly, medial to the ophthalmic division of the trigeminal nerve to supply the dura of the inferior lateral wall of the cavernous sinus and the nerves related to the lateral wall of the sinus. It also supplies the area around the foramina ovale and spinosum and may anastomose with branches of the middle meningeal artery.[15] The capsular arteries of McConnell arise from the medial aspect of the horizontal segment and are distributed to the anterior and inferior aspects of the pituitary gland and to the dura of the sellar floor. These arteries are difficult to demonstrate in regular anatomical dissections.
The Clinoid Segment
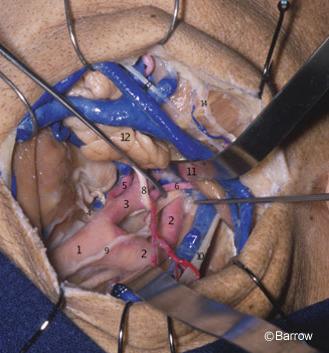
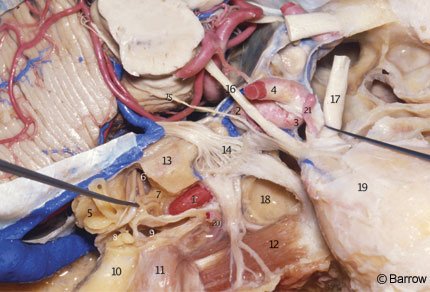
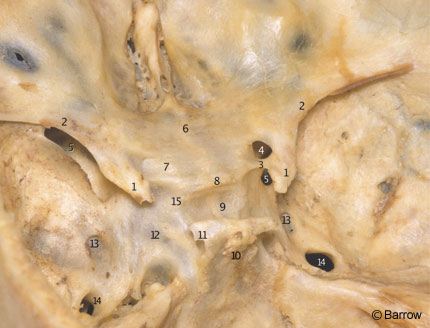
The clinoid segment of the ICA relates closely with the anterior clinoid process. In fact, the anterior clinoid process must be removed to expose the clinoid segment of the ICA (Fig. 14). It lies medial to the anterior clinoid process and is surrounded by two distinct rings. The more proximal ring forms the actual roof of the cavernous sinus. This tenuous connective tissue membrane was described by Nutik[13] and called the carotid-oculomotor membrane by Inoue et al.[8] It extends from the lateral side of the ICA to the medial surface of the oculomotor nerve and then to the posterior clinoid process. It surrounds the anterior vertical segment of the ICA and forms a roof to the space lateral to the carotid artery and medial to the oculomotor nerve in the anterior portion of the roof of the cavernous sinus. This membrane separates the venous contents of the cavernous sinus from the virtual space occupied by the anterior clinoid process. It is continuous with the inner reticular layer that envelops the fourth cranial nerve and the ophthalmic branch of the trigeminal nerve on the lateral wall of the cavernous sinus (Fig. 15).
The dura covering the base of the skull extends anteriorly to the anterior clinoid process and tightly envelops the ICA at its entrance into the subarachnoid space. It forms the so-called distal ring, originally described by Perneczky et al.[16] who termed it the fibrous ring. From a practical perspective, the dura related to the distal ring is “fused” with the adventitial wall of the ICA (Fig. 15). No attempt should be made to dissect it from the arterial wall. Rather, it should be incised close to the artery when the proximal ICA needs to be mobilized or when proximal ICA aneurysms must be fully exposed.The dura surrounding the distal ring is related to bony structures in most of its circumference (i.e., the anterior clinoid process posteriorly, superiorly, and laterally; the optic strut anteriorly, superiorly, and medially; and the middle clinoid process inferiorly and medially, Fig. 14). Its posteromedial aspect, exactly where the carotid cave occurs, however, is devoid of any bony relationships.[7]
The distal portion of the carotid sulcus that runs in the lateral aspect of the body of the sphenoid bone and contains the cavernous ICA usually is proximal to the distal ring.[7] The distal ring has no horizontal orientation on the axial plane but is inclined in a posteromedial direction.[14] If divided into two halves, the medial half would be located more inferiorly and posteriorly than the lateral half. This information is useful when planning surgery to treat paraclinoid aneurysms because the surgical strategy depends on the relation of the aneurysm to the distal ring.
It is not yet possible to predict the precise location of the distal ring by radiological methods.[10] In an anatomical and radiological study, Oikawa et al.[14] found that the medial edge of the distal ring was situated slightly superior to the tuberculum sellae, and the lateral edge was located inferior to the superior border of the anterior clinoid process when viewed from a lateral radiographic projection. However, some medial edges were located even lower than the tuberculum sellae. They pointed out that aneurysms with such a proximal origin may be associated with a relatively high risk of causing subarachnoid hemorrhage.
The supraclinoid ICA enters the subarachnoid space lateral to the optic nerve and medial to the anterior clinoid process. The dura forming the distal ring is contiguous medially with the dura of the diaphragm sellae and laterally with the dura that envelops the anterior clinoid process. The anterior clinoid process is situated at the medial end of the lesser sphenoid wing, in the anterior part of the roof of the sinus, and forms the lateral wall of the intracranial end of the optic canal (Fig. 14). The anterior clinoid process is usually solid, but it can eventually be pneumatized and communicate via the optic strut with the sphenoid sinus located at the medial wall of the cavernous sinus (Fig. 15). The presence of such air cells should be inspected in anterior clinoidectomies to prevent a postoperative CSF fistula from developing.
The anterior clinoid process may be connected with the posterior clinoid process by a fibrous or osseous bridge that can make its removal difficult. Also the anterior and middle clinoid processes may by united by a bridge of bone, forming an ostium called the caroticoclinoid foramen, at the distal end of the clinoid segment of the ICA. During surgical procedures for pathologies that involve the cavernous sinus and paraclinoid region, resection of the anterior clinoid process and section of the distal dural ring are important steps in providing a site for proximal control of arterial bleeding (e.g., aneurysms arising at the ophthalmic segment of the ICA). It also allows mobilization of the anterior vertical portion of the ICA.[2-4,10,12,13,16,20]
Debate exists about the actual relationshipof the clinoid segment of the ICA to the cavernous sinus. At present, we can state that this segment is extradural. Detailed anatomical studies[10,19] have demonstrated venous blood surrounding, to variable degrees, the clinoid ICA, because the proximal dural ring of the ICA is incompetent. Although enveloping the clinoid ICA in a collar-like fashion, it does not tightly adhere to the arterial wall and allows venous channels to pass between the collar and the arterial wall. We assume that the clinoid venous plexus surrounding the ICA is an actual extension of the anterior venous compartment of the cavernous sinus (Fig. 15). We find no evidence to suggest that this venous plexus has an origin other than the cavernous sinus. An elegant description of the arrangement of the dural layers around the clinoid segment of the ICA is provided by Seoane et al.[19]
In 1989 Kobayashi et al.[11] introduced the term carotid cave. Frequently misinterpreted, this term is used to describe a small recess or pouch of dura mater on the medial side of the wall of the ICA at the level of the distal dural ring (Fig. 16). Hitotsumatsu et al.[7] studied the microsurgical anatomy of the carotid cave bilaterally in 25 anatomical specimens. They found the carotid cave in 34 of 50 sides (68%), including four caves that were recognized only after microscopical or histological verification, an observation supported by Kim et al.[10] They observed that the morphology of the cave was variable and distinguished three configurations according to the topographic microanatomy of the cave: a slit-type (34%), a pocket-type (24%), and a mesh-type (10%). Thirty-two percent of the specimens showed a tight dural attachment with no cave-like structure around the distal dural ring. Interestingly, the cave occurred at the posteromedial aspect of the artery, where the distal ring has no contact with any bony structure.
Based on these observations, the authors suggested that so-called carotid-cave aneurysms project medially and posteriorly on anteroposterior and lateral angiograms, respectively. The neck of the carotid cave aneurysm should be seen just below or proximal to the level of the tuberculum sellae on lateral angiograms. It is also noteworthy that superior hypophyseal arteries may originate in the carotid cave. The carotid cave has not been observed on the lateral surface of the ICA. Certainty regarding the exact position of proximal and medial ICA aneurysms, especially small ones, may be extremely difficult to establish. Surgical inspection may be required to determine the actual location of the aneurysm at the level of the carotid cave or inside the cavernous sinus. The management of such lesions varies according to the location of the aneurysm. Small aneurysms inside the cavernous sinus are usually treated conservatively.
Conclusions
The course of the carotid artery anatomy and its adjacent structures as it ascends from the neck toward the skull base and then proceeds toward the subarachnoid space is extremely complex. A clear three-dimensional understanding of its microsurgical anatomy is required to perform surgical approaches directed to various segments of the ICA.
Acknowledgment
This work was supported by the Steele Foundation.
References
- Bouthillier A, van Loveren HR, Keller JT: Segments of the internal carotid artery: A new classification. Neurosurgery 38:425-433, 1996
- de Oliveira E, Tedeschi H, Rhoton AL, Jr.: Microsurgical anatomy of the internal carotid artery: Intrapetrous, intracavernous, and clinoidal segments, in Carter Lp, Spetzler RF (eds): Neurovascular Surgery. New York: McGraw-Hill, Inc., 1994
- Dolenc VV: A combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg 62:667-672, 1985
- Dolenc VV, SkrapM, Sustersic J, et al: A transcavernous-transsellar approach to the basilar tipaneurysms. Br J Neurosurg 1:251-259, 1987
- Fischer E: Die Labeabweichungen der vorderen hirnarterie im Gefalblid. Zentrallbl Neurochir 3:300-313, 1938
- Gibo H, Lenkey C, Rhoton AL, Jr.: Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg 55:560-574, 1981
- Hitotsumatsu T, Natori Y, Matsushima T, et al: Micro-anatomical study of the carotid cave. Acta Neurochir (Wien) 139:869-874, 1997
- Inoue T, Rhoton AL, Jr., Theele D, et al: Surgical approaches to the cavernous sinus: A microsurgical study. Neurosurgery 26:903-932, 1990
- Katsuta T, Rhoton AL, Jr., Matsushima T: The jugular foramen: Microsurgical anatomy and operative approaches. Neurosurgery 41:149-202, 1997
- Kim JM, Romano A, Sanan A, et al: Microsurgical anatomic features and nomenclature of the paraclinoid region. Neurosurgery 46:670-682, 2000
- Kobayashi S, Kyoshima K, Gibo H, et al: Carotid cave aneurysms of the internal carotid artery. J Neurosurg 70:216-221, 1989
- Matsuyama T, Shimomura T, Okumura Y, et al: Mobilization of the internal carotid artery for basilar artery aneurysm surgery. Technical note. J Neurosurg 86:294-296, 1997
- Nutik SL: Removal of the anterior clinoid process for exposure of the proximal intracranial carotid artery. J Neurosurg 69:529-534, 1988
- Oikawa S, Kyoshima K, Kobayashi S: Surgical anatomy of the juxta-dural ring area. J Neurosurg 89:250-254, 1998
- Paullus WS, Pait TG, Rhoton AL, Jr.: Microsurgical exposure of the petrous portion of the carotid artery. J Neurosurg 47:713-726, 1977
- Perneczky A, KnospE, Vorkapic p, et al: Direct surgical approach to intraclinoidal aneurysms. Acta Neurochir (Wien) 76:36-44, 1985
- Quisling RG, Rhoton AL, Jr.: Intrapetrous carotid artery branches: Radioanatomic analysis. Radiology 131:133-136, 1979
- Rhoton AL, Jr.: Absence of bone over the geniculate ganglion. J Neurosurg 28:48-53, 1968
- Seoane E, Rhoton AL, Jr., de Oliveira E: Microsurgical anatomy of the dural collar (carotid collar) and rings around the clinoid segment of the internal carotid artery. Neurosurgery 42:869-886, 1998
- Seoane E, Tedeschi H, de Oliveira E, Wen HT, Rhoton AL, Jr.: The pretemporal transcavernous approach to the interpeduncular and prepontine cisterns: Microsurgical anatomy and technique application. Neurosurgery 46:891-898, 2000.
- Suttner N, Mura J, Tedeschi H, et al: Persistent trigeminal artery: A unique anatomic specimen—analysis and therapeutic implications. Neurosurgery 47:428-434, 2000
- Yasargil MG: Microneurosurgery. New York: Georg Thieme Verlag, 1984
