
Craniectomy for Traumatic Brain Injury
Authors
Steve Chang, MD
Timothy R. Harrington, MD
Scott R. Petersen, MD*
Divisions of Neurological Surgery and *Trauma Surgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
Several neurotrauma centers, including ours, routinely use decompressive craniectomy to treat patients with a severe traumatic brain injury. Whether craniectomy improves outcomes, however, remains controversial. Studies have failed to show that craniectomy offers a clear advantage compared to conservative treatments. Attempts to determine the utility of decompression have been insufficient and have not been evidence based. Recently, encouraging class II data have begun to spur interest in the procedure. Although the findings are promising, a multicenter prospective randomized trial is needed to provide clinicians with clear criteria for surgical decompression of the brain.
Key Words: craniectomy, intracranial pressure, traumatic brain injury
Injuries to the brain are the most common cause of trauma-related death and disability. Among the many advances in neurological critical care, the realization of the need to prevent secondary insults to the brain has dictated treatment strategies. In 1996 a landmark publication sponsored by the Brain Trauma Foundation, the Guidelines for the Management of Severe Head Injury,[1] provided evidence-based standards, guidelines, and options that have helped trauma personnel to recognize that secondary insults can be limited or exacerbated, depending on the treatment that patients receive after injury. These guidelines were refined and upgraded in 2000.[2]
After traumatic brain injury (TBI) itself, secondary brain injury is the leading cause of hospital deaths. [10] Recognizing the prominent role of secondary brain damage in the overall deaths and complications of TBI patients has contributed to the decline in the mortality rates associated with TBIs in recent studies. [5] In the 1970s, a 55% mortality rate was common among unmonitored patients.[6] With the advent of critical care, routine computed tomography (CT), and monitoring of intracranial pressure (ICP), this rate improved to about 30%.[6] In the past few years, mortality rates have been in the range of 20% when ICP and cerebral perfusion pressure have been managed carefully.[6]
Still, proven therapies for brain injuries are lacking. Several major clinical trials have found neuroprotective agents to be ineffective.[3,13] Given these failures, interest in early decompressive surgery has been renewed. Several trials have evaluated the procedure with promising results.[4,12] A preliminary trial to evaluate decompressive surgery is under study, and several neurotrauma centers, including our own, routinely use this method of brain decompression to treat appropriate injuries. To illustrate the current management of TBIs at our institution, we present a patient with a TBI who was treated with a decompressive hemicraniectomy.
Case Report
A 17-year-old, previously healthy male was transferred to our institution after falling from the back of a moving pickup truck. His initial CT scan showed right frontal and right temporal intracranial contusions without evidence of midline shift or extraaxial collections. There were no associated extracranial injuries.
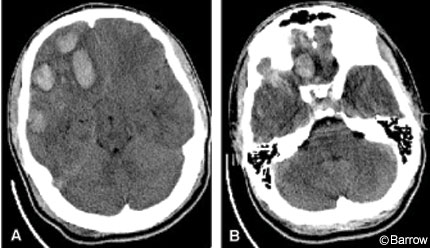
On arrival at our institution, the patient’s initial Glasgow Coma Scale (GCS) score was 13 (eye response, 3 points; motor response, 6 points; verbal response, 4 points) with equal and reactive pupils. CT of the head confirmed the presence of multiple contusions without cisternal effacement and a 0.4-cm midline shift (Fig. 1). The patient’s high level of neurological function and ability to follow commands allowed rapid serial neurological examinations. Initially, conservative medical management was elected. On the third day of hospitalization, the patient’s neurological status declined, prompting repeat scanning (Fig. 2). Serial head CT showed increasing mass effect, especially in the middle fossa. The decision was made to perform a decompressive hemicraniectomy.
The patient underwent a right frontotemporoparietal hemicraniectomy with a slit durotomy. A large craniotomy bone flap was turned and extended to the floor of the temporal fossa using rongeurs. The dura was opened with a No. 15 blade parallel to the temporal lobe. A small amount of the temporal lobe protruded, but there was no gross herniation. Multiple horizontal slit incisions were made in the dura about 3 cm to the right of the superior sagittal sinus and extending down to the temporal lobe. The dura was covered with DuraGen dural graft matrix (Integra LifeSciences Corporation, Plainsboro, NJ). A subgaleal drain and a Codman ventricular catheter (Johnson & Johnson Professional Inc., Raynham, MA) were placed intraoperatively to enable ICP to be monitored. The galeal layer was closed with multiple 2-0 vicryl sutures, and the skin was closed with staples.
Abdominal placement of a bone flap was elected, and a transverse incision was made in the right periumbilical region. Dissection extended to the rectus abdominis fascia. A large subcutaneous pocket was made where the bone flap was placed. The incision was closed with multiple 3-0 vicryl interrupted sutures in the subcutaneous layer, and the skin was closed with staples.
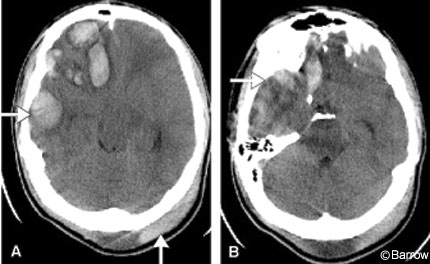
Postoperatively, the patient did well and CT demonstrated good decompression (Fig. 3). In the recovery room his ICP was 2 to 3 mm Hg. In the neurologic critical care unit, his ICP increased to 20 mm Hg only once. One dose of mannitol was given; otherwise, his ICP remained in the normal range. By postoperative Day 2, the patient began to follow commands and was oriented to his name and date. The next day ICP monitoring was discontinued. On postoperative Day 5, he was transferred from the intensive care monitoring unit to a floor unit.
On postoperative Day 7, a pseudomeningocele associated with drainage from his cranial incision developed. It was managed conservatively with the wound oversewn. Cultures were positive for Staphylococcus, and intravenous nafcillin and rifampin were administered. A subgaleal drain replaced on postoperative Day 9 and left in place for 5 days successfully decompressed the pseudomeningocele. There was no further drainage, and the surrounding edema and erythema resolved. The patient was admitted to the inpatient neurorehabilitation unit and discharged home on hospital Day 28.

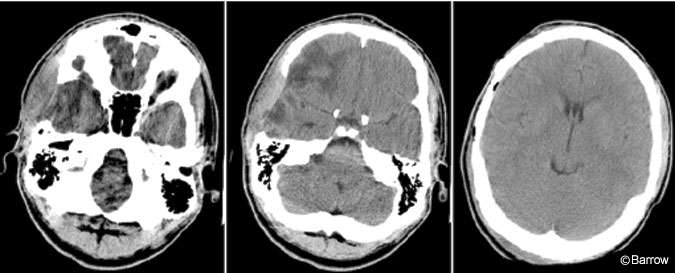
Three months after surgery the patient returned to have his abdominal bone flap removed and his cranial defect repaired. The bone flap was well vascularized and did not appear infected (Fig. 4). The flap was soaked in antibiotic solution and replaced in the routine fashion without complications. The patient tolerated the procedure well and his postoperative GCS score was 15. At the time of writing, he had resumed his academic pursuits.
Discussion
The European Brain Injury Consortium (EBIC) and the American Association of Neurological Surgeons (AANS) guidelines mention various second-tier therapies for the treatment of TBI:[1,8] barbiturate coma, forced hyperventilation, mild hypothermia, and surgical decompression. However, there are several questions regarding surgery for patients with a TBI. Is decompressive craniectomy beneficial for all TBI patients or only for selected subgroups of patients? What are the indications? Which surgical technique should be used? What is the optimal timing of surgery?
Currently, no class I data support the conjecture that decompressive craniectomy improves outcomes in unselected TBI patients. Although the procedure was described as early as the 1800s,[9] class III retrospective studies have found no benefit associated with its use.[12] The reasons are multifactorial. Inevitably, reserving a decompressive craniectomy as a salvage operation may render it too late to offset irreparable secondary ischemic damage.
Recently, however, prospectively analyzed class II data have offered support for craniectomy as a treatment for TBI. A review of the available class II studies found that about 70% of patients undergoing decompressive craniectomy survived the operation.[14] Two thirds had a good outcome, a finding far better than that associated with barbiturate coma. For example, 27 children with increased ICP who failed conservative therapy were followed prospectively.[15] Glasgow Outcome Scale scores 6 months after injury were favorable in 7 of the 13 decompressed patients compared to only 2 of the 14 patients in the control group. Unfortunately, the decompressive craniectomy involved only bone removal; the dura was not enlarged.
Between 1997 and 1999, Meier et al.[11] decompressed 19 of 128 patients for intractable brain swelling. Of these 19 patients, 17 underwent bilateral craniectomy including enlargement of the dura. Good outcomes were achieved in 26% of the patients. The mortality rate was considerably lower in patients with isolated head injuries than in patients with multiple trauma (27% versus 63%.)
In a pilot study, Coplin et al.[4] compared the feasibility of craniectomy with duraplasty to the standard surgical procedure in comatose patients with a midline shift not explained by removable hematoma. The choice of surgical approach was left to the surgeon: a standard approach with evacuation of space-occupying lesion or frontal or temporal lobectomies with primary dural closure. Twelve patients underwent decompressive craniectomy surgery and 17 patients underwent standard surgery. Craniectomy and augmentative duraplasty were intended to increase the volume of space available for expansion of edematous brain tissue and thus to prevent impedance of cerebral blood flow and secondary ischemia. Both groups had similar outcomes even though patients in the decompressed group had lower admission GCS scores and worse CT scans as judged according to the Traumatic Coma Data Bank scoring system. In the group that underwent the standard surgery, three patients (18%) were returned to the operating room for control of ICP. In contrast, no patient in the craniectomy group was returned to the operating room for intracranial surgery other than for replacement of the skull defect. Although not statistically significant, the mortality rate tended to decrease while neurologic outcomes of survivors in both groups were equivalent. These results were achieved even though the craniectomy group had more severe injuries than the group receiving standard surgical treatment as measured by GCS score at the time of initial surgery and by worse findings on CT. These results were encouraging, but this observational study had major shortcomings: Patients were not randomized to the different surgical modalities, and each surgeon performed the procedure deemed in the patient’s best interest.
The current role of decompressive craniectomy in neurotrauma is limited (<3% in severe TBI).[7] The guidelines established by the AANS cite decompressive craniectomy as the last of the second-tier therapeutic options. Given the promising results of prospective studies, however, this simple and safe procedure is gaining popularity as the most promising therapy for patients with refractory ICP.
Whether prophylactic decompression based on clinical signs (i.e., neurological state, CT) improves outcomes remains open. Multiple factors worked in favor of our patient. First, there is consensus that the younger a patient is with a TBI, the better the outcome of decompressive craniectomy will be. No convincing data, however, favor a particular age limit.[7,11,12] Second, our patient had a high initial GCS score, an established positive prognostic factor for TBI. He also had no other traumatic injuries and thus, was an ideal candidate for decompression.
Conclusions
Although class I studies are lacking, evidence from prospective, uncontrolled trails indicates that decompressive craniectomy improves outcomes. The present case adds to the literature that supports the procedure as well tolerated and associated with the potential for great benefit. Prospective, controlled, randomized studies are being conducted to define the effect of this well-described neurosurgical procedures on the outcome of patients with TBI.
References
- Brain Trauma Foundation, American Association of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care: Guidelines for the management of severe head injury. J Neurotrauma 13:641-734, 1996
- Brain Trauma Foundation, American Association of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care: Part 1: Guidelines for the management of severe traumatic brain injury (abstract). J Neurotrauma 17:457-627, 2000
- Bullock MR, Lyeth BG, Muizelaar JP: Current status of neuroprotection trials for traumatic brain injury: Lessons from animal models and clinical studies. Neurosurgery 45:207-220, 1999
- Coplin WM, Cullen NK, Policherla PN, et al: Safety and feasibility of craniectomy with duraplasty as the initial surgical intervention for severe traumatic brain injury. J Trauma 50:1050-1059, 2001
- Elf K, Nilsson P, Enblad P: Outcome after traumatic brain injury improved by an organized secondary insult program and standardized neurointensive care. Crit Care Med 30:2129-2134, 2002
- Ghajar J: Traumatic brain injury. Lancet 356:923-929, 2000
- Guerra WK, Gaab MR, Dietz H, et al: Surgical decompression for traumatic brain swelling: Indications and results. J Neurosurg 90:187-196, 1999
- Maas AI, Dearden M, Teasdale GM, et al: EBIC-guidelines for management of severe head injury in adults. European Brain Injury Consortium. Acta Neurochir (Wien) 139:286-294, 1997
- Marcotte CA: De L’Hemicraniectomie Temporaire (thesis). Paris: University of Paris Medical Faculty, 1896
- Marshall LF, Gautile T, Klauber MR: The outcome of severe closed head injury. J Neurosurg 75:28-36, 1991
- Meier U, Zeilinger FS, Henzka O: The use of decompressive craniectomy for the management of severe head injuries. Acta Neurochir Suppl 76:475-478, 2000
- Munch E, Horn P, Schurer L, et al: Management of severe traumatic brain injury by decompressive craniectomy. Neurosurgery 47:315-323, 2000
- Narayan RK, Michel ME, Ansell B, et al: Clinical trials in head injury. J Neurotrauma 19:503-557, 2002
- Peik J: Decompressive surgery in the treatment of traumatic brain injury. Curr Opin Crit Care 8:134-138, 2002
- Taylor A, Butt W, Rosenfeld J, et al: A randomized trial of very early decompressive craniectomy in children with traumatic brain injury and sustained intracranial hypertension. Childs Nerv Syst 17:154-162, 2001
