
Auditory Brainstem Implantation
Gregory P. Lekovic, MD, PhD, JD
L. Fernando Gonzalez, MD
Mark J. Syms, MD*
C. Phillip Daspit, MD*
Randall W. Porter, MD
Division of Neurological Surgery and *Section of Neurotology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
Auditory brainstem implants (ABIs) can restore meaningful hearing to patients deafened by neurofibromatosis type 2. These devices are similar to cochlear implants on which their design is based. Current multichannel ABI technology affords a level of performance in aiding speech comprehension similar to that offered by single-channel cochlear implants. ABIs differ from cochlear implants in that the brainstem implants stimulate the cochlear nuclei directly, bypassing the cochlear nerve. Multichannel ABIs can be implanted at the time of first or second side surgery for tumors involving the auditory nerve. Most surgeons favor a translabyrinthine approach for ABI placement, because the stimulating leads need to be implanted within the lateral recess of the fourth ventricle. At this location, the ventral cochlear nucleus can be stimulated along its lateral to medial axis. Intraoperative monitoring of brainstem auditory evoked potentials is essential and helps confirm adequate positioning of the device. After programming, about 85% of patients experience auditory percepts. Only a relatively rare, but significant, minority of patients is able to attain sound-only open-set speech comprehension with their ABI. Most experience a significant improvement in open-set speech comprehension when used as an adjunct to lipreading. Speech comprehension continues to increase as long as 8 years after device implantation. More than 90% of recipients use their ABI daily. Most are satisfied with their decision to obtain an ABI and would recommend the device to others.
Key Words: acoustic neuroma, auditory brainstem implantation, auditory prosthesis, neurofibromatosis type 2, vestibular schwannoma
Since 1979 auditory brainstem implants (ABIs) have provided prosthetic hearing for patients deafened by neurofibromatosis-2 (NF-2). The first ABI was a comparatively primitive, single-channel device that aided users by increasing awareness of environmental sounds and lipreading. Contemporary multichannel ABIs afford a level of performance comparable to that achieved with single-channel cochlear implants, that is, the ability to discriminate environmental sounds, marked improvement in lipreading, and limited openset speech comprehension. Some ABI users can even communicate on the telephone. This article reviews the development of ABIs, emphasizing relevant neuroanatomical and physiological principles, intraoperative technique, related surgical anatomy, clinical outcomes, and future directions for research.
History of ABIs
At the time of this writing, more than 400 ABIs have been placed worldwide.[17] The first ABI implant was a simple ball electrode implanted into the parenchyma of the cochlear nucleus at the House Ear Institute in 1979. This electrode migrated from its implantation site, and the design was soon modified into a pair of platinum plates with a synthetic mesh backing. Subsequent iterations of ABI design culminated in the development of multichannel implants composed of 8 to 21 platinum discs embedded in plastic with a synthetic mesh backing, depending on the manufacturer or model.
In 1994 a clinical trial was begun in the United States to evaluate the safety and efficacy of multichannel ABIs. This trial was concluded in 2000 and culminated in the Food and Drug Administration (FDA) approving the Nucleus 24 Contour (Cochlear Corporation, Englewood, CO) and Nucleus 22 (Cochlear Corporation, Englewood, CO) for implantation via the translabyrinthine approach for the treatment of deafness related to NF-2. Currently, the Nucleus 24 is the most widely implanted ABI in the world, including North America, Australia, and the European Union. Other available ABIs include the MXM Digisonic (Laboratories MXM, Valaurious Cedex, France), Med-EL (Med EL Corporation, Research Triangle Park, NC), and Clarion (Advanced Bionics, Sylmar, CA). So far no evidence suggests that the efficacy of the various multichannel ABIs differs.
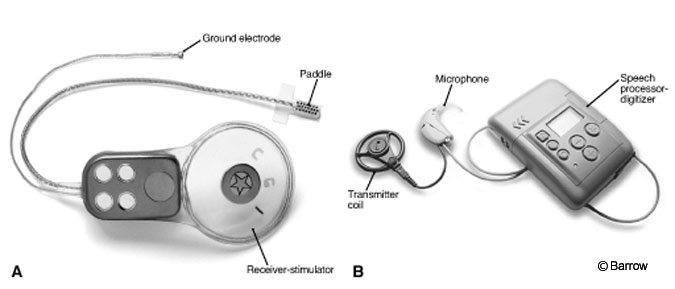
Design of ABIs
All ABIs share certain features regardless of specifications (Fig. 1). The basic electrode itself is placed within the lateral recess of the fourth ventricle and connected via a cable implanted into a groove in the temporal bone to a receiver-stimulator. The latter is seated in the bone of the skull behind the helix of the ear above the canthomeatal line. A second component consists of microphone, speech processor, and radiotransmitter coil that detects sound, converts it to a digital signal, and transmits it to the receiver, respectively. The transmitter attaches to the receiver-stimulator by a magnet. Once an ABI is implanted, monopolar cauterization is strictly forbidden from use for fear of damaging the electrode, brain, or both. Recently, the magnet has been replaced with a nonmagnetic plug at surgery, and the transmitter coil is fixed to the scalp with adhesive. These changes permit MR imaging from the outset.
ABIs are not activated until a patient recovers from surgery. Because of concerns about the potential activation of brainstem structures, many centers prefer that the electrode be activated in a monitored setting. After successful ABI implantation, medical and audiological follow-up is performed at least every 3 months for the first year and then annually thereafter.
Indications for ABIs
The FDA has approved the ABI for patients with NF-2 who are 12 years or older who have reasonable expectations and motivation. The degree of hearing loss is not a deciding factor (i.e., there is no audiological cut-off for ABI placement). Other factors also distinguish optimal ABI candidates: good overall health, acceptable vision (because the ABI may aid most with lipreading), high motivation, excellent family support, acceptable anatomical status, and an interest in spoken communication. Because the ABI creates an ‘unnatural’ quality of sound, many users are disappointed with it at first. High motivation and excellent family support are important to ensure that users do not drop out of the ABI program.
These issues are exemplified in the House experience with placing ABIs in teenagers. 17 Of 21 teens implanted, four dropped out of the program. One later returned and has become an above average ABI user as a result of strong family support and encouragement. Qualities most characteristic of program dropouts included low motivation and poor family support. However, an important lesson from the House experience has been that the ideal candidate is self-motivated to hear again rather than motivated for the benefit of family members.
The only absolute contraindication to the placement of an ABI is an active infection. Relative contraindications are rare and include patients who may meet the formal criteria but who lack adequate support or motivation, as outlined above. As discussed later, if the cochlear nerve can be preserved intact after resection of an acoustic neuroma, placement of a cochlear implant may be indicated if promontory testing is positive.
The FDA has approved the use of ABIs only in the setting of NF-2. However, off-label use of the device has been reported (predominantly in Europe) for other indications, including deafness related to bilateral skull base trauma,[3] sporadic acoustic neuroma in patients with only one hearing ear,[4] cochlear nerve hypoplasia or aplasia,[2,5] cochlear ossification, [11] bilateral auditory nerve deafness,[22] or involvement with other neoplastic syndromes such as Bourneville’s disease and von Hippel-Lindau disease.[15]
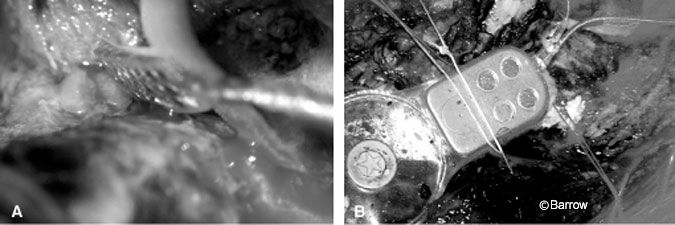
Since ABIs were approved by the FDA, they have been successfully implanted at an increasing number of domestic and international centers. In October 2004, the first ABI was placed at our institution (Fig. 2).
First Side vs. Second Side Surgery
Initially, ABIs were placed at the time of second side surgery for tumor removal. Now more than a third of ABIs are placed during the first surgery (i.e., while the patient still has serviceable hearing on the contralateral side).[18]
There are several advantages to placing the device during tumor removal on the first side. If the device is improperly positioned within the lateral recess of the fourth ventricle, does not elicit auditory sensations, or creates intolerable side effects that prevent its use, then the contralateral side can be implanted when surgery is performed on the second side. The patient thereby has a second chance to obtain a functioning implant. Conversely, if the first side is implanted and functions well, the user can become accustomed to the device while still able to hear with the other ear. This situation may help the patient to acclimatize to and interpret the ABI sound. However, most patients implanted during surgery on the first side do not become daily users of ABI until their deafness is bilateral.
Promontory Testing
Promontory testing is the direct stimulation of the cochlear promontory via an electrode placed surgically in the middle ear. The procedure is performed under local anesthesia through a small myringotomy and can distinguish between cochlear and retrocochlear causes of deafness. In a negative promontory test, pitch is not perceived in response to direct electrical stimulation of the cochlea. The finding implies a retrocochlear cause of deafness. Conversely, in a positive test, pitch is perceived in response to stimulation, indicating that the cochlear nerve is capable of transmitting tonal information centrally.
Marangos et al.[15] examined 19 ears with promontory testing in patients with profound bilateral deafness. The promontory tests were positive in five of six ears that had not undergone previous surgery and in three other surgically treated ears with residual tumor. The patient who responded negatively on promontory testing but who had undergone no previous surgery had bilateral acoustic neuromas causing profound brainstem compression. A patient presenting with deafness one year after undergoing a subtemporal approach in which hearing was preserved had a positive promontory test. This patient was fitted with a cochlear implant and had an excellent response.
Marangos et al.[15] concluded that the goal of first-side surgery should be to preserve hearing or at least the anatomical integrity of the auditory nerve to identify patients who will respond to cochlear implantation. Preservation of the cochlear nerve depends on the size of the tumor. Small tumors are usually found in patients with a known family history of NF-2. In sporadic cases, the tumors are often diagnosed when too large for the cochlear nerve to be preserved. They further argued that ABI should be reserved for patients with a negative preoperative promontory test, patients in whom the cochlear nerve cannot be preserved, or patients whose deafness is both cochlear and retrocochlear (e.g., related to tumor infiltration of the auditory nerve).
Neuroanatomy and Physiology of Audition
Sound is represented spatially in the auditory system. This spatial relationship, called tonotopy, is maintained with fidelity in ascending neuroanatomical pathways from the peripheral auditory end organ (the organ of Corti in humans) to the primary auditory cortex. In the organ of Corti, hair cells line the basal membrane of the cochlea in a tonotopic fashion. Hair cells responding best (i.e., with a characteristic frequency) to high pitches are located at the basal turn of the cochlea, and hair cells responding best to low frequencies are located at the cochlear apex.
In his theoretical work on the nature of music, Tonemfindungen, Helmholtz proposed that sound delivers a standing wave to the basement membrane and that regionalized movements of this structure responded to specific frequencies to determine tonotopy in the cochlea. This model predicts that the hair cells of the cochlea are passive transducers of frequency. Although still widely taught, this view is now understood to be incomplete. In fact, hair cells are dynamic, and various adaptations, including active motor processes and electrical tuning, determine their characteristic frequency. These adaptations tune the hair cells, such that they preferentially depolarize in response to their characteristic frequency. Loud sounds, however, flatten their tuning curve.
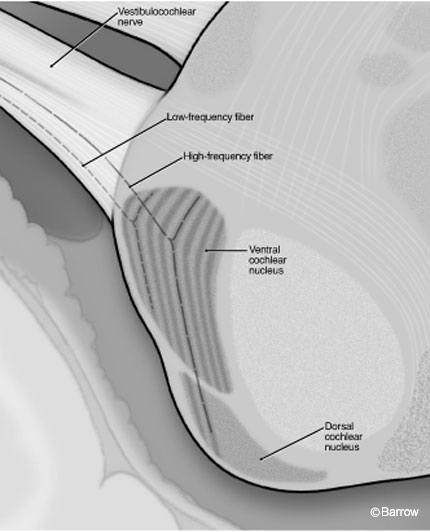
To preserve this tonotopic relationship, ascending primary afferents of the spiral ganglion must maintain fidelity to the characteristic frequency of the inner hair cells that they supply. Thus the cochlear division of the vestibulocochlear nerve is composed of afferent fibers, each of which represents a specific pitch-place within the cochlea. As these fibers enter the brainstem, they terminate in regions of the cochlear nucleus that again reflect spatial segregation of frequency encoding. Specifically, afferents with a high characteristic frequency (in humans the upper limit of hearing is about 20 kHz) plunge deep into the ventral cochlear nucleus before bifurcating and sending a descending branch to the dorsal cochlear nucleus. In contrast, low-frequency fibers bifurcate superficially within the substance of the ventral cochlear nucleus. All primary afferent fibers bifurcate within the ventral cochlear nucleus before terminating.
If characteristic frequency is plotted against position in the brainstem, the cochlear nuclei are composed of isofrequency laminae, or layers of neurons (like the layers of an onion) (Fig. 3). In this tonotopic organization, the superficial regions of the cochlear nuclei (the lateral and anterior laminae of the anterior and posterior divisions of the ventral cochlear nucleus) represent low frequency sounds, whereas the deeper regions of the nucleus represent higher frequencies. Unfortunately, the aspect of the cochlear nuclei accessible to surface electrode stimulation is therefore restricted to a very narrow range of frequencies. This limitation is the motivation behind the development of penetrating electrodes for implantation.
In addition to the tonotopic organization of the cochlear nuclei, the rich constellation of neuronal cell types and interconnections is only partly understood. Many ABI recipients are able to perceive pitch. Most speech is comprehensible over a relatively narrow band of frequencies. Although pitch ranking of multiple channels is thought to facilitate speech comprehension, speech comprehension may be improved with multiple channels even if pitch ranking is impossible, and the extent of pitch ranking does not necessarily correlate with ABI performance outcomes.[13] The cochlear nuclei are composed of many different neuronal populations, including fusiform cells, globular and spherical bushy cells, and octopus cells, among others. Many neuronal cell types within the cochlear nuclei are specialized to respond to information other than strictly pitch. For example, some cells respond best to the onset of a sound stimulus, others to the cessation of such a stimulus. This specialization may explain why multiple channels may improve speech comprehension even if the patient is unable to use the channels to recognize different pitches. Alternatively, if improved speech comprehension requires such ‘on’ and ‘off’ signals or other more complicated stimuli, then strategies such as penetrating ABIs that focus on the tonotopic organization of the cochlear nuclei may not improve speech comprehension.

Surgical Considerations and Techniques
Surgical Anatomy
The surgical goal of ABI implantation is to position the electrode array on the surface of the cochlear nuclei in the lateral recess of the fourth ventricle (Fig 4). The landmarks for proper identification of the lateral recess include the origins of the facial, vestibulocochlear, glossopharyngeal, and vagus nerves; the flocculus of the cerebellum, the choroid plexus exiting the foramen of Luschka, and the taenia. However, these landmarks can be severely distorted by tumor.
The simplest way to identify the foramen of Luschka is to follow the vestibulocochlear nerve proximally. When the nerve is cut, its stump can be identified and similarly followed. In a study of the cerebellopontine angle in 100 human specimens, the distance between the facial and vestibulocochlear nerves was 4.7 mm ± 0.9 mm.12 The distance between the vestibulocochlear and glossopharyngeal nerves was 5.5 mm ± 1.0 mm. Thus, if the root of the vestibulocochlear nerve itself cannot be identified with confidence, its root lies in a triangle approximately 5 x 6 mm within the origins of the facial and glossopharyngeal nerves.
In the same study[12] the dimensions of the foramen of Luschka were about 3.4 mm x 2.0 mm. The foramen was wide open in only 24% of cases, open only after incision of the arachnoid in 53%, functionally closed (requiring extensive dissection) in 18%, and anatomically occluded in 5%. The taenia of the choroid was present in 92% of specimens, and it required incision to access the lateral recess in 51%.
Importantly, Matthies et al.[16] analyzed the outcomes of ABI placement as a function of the amount of dissection required to identify and open the foramen of Luschka. There was no relationship between the density of arachnoid adhesions involving the foramen of Luschka and outcomes with the ABI. However, these data were obtained from a small sample (n = 8) and may lack the power to demonstrate such a relationship.
The cochlear nucleus extends from the floor of the fourth ventricle medially to the entry zone of the vestibulocochlear nerve at the ventrolateral brainstem surface. In an anatomical study of the cochlear nuclei of humans, Quester and coworkers[19,20] made thread measurements of the length of the cochlear nucleus complex from the auditory tubercle (which overlies the dorsal cochlear nucleus) to the entry zone of the vestibulocochlear nerve. The length of the cochlear nucleus was 12.8 mm ± 1.2 mm. The length of the nucleus located within the lateral recess (i.e., primarily the ventral cochlear nucleus and site of ABI placement) was 7.6 mm ± 1.5 mm. Thus the dimensions of current generations of ABI (i.e., about 3 x 8 mm) are well suited to placement of the device completely within the lateral recess.
Surgical Approach
The original clinical trial protocol called for ABI placement via a translabyrinthine approach, which remains the most popular approach. The translabyrinthine approach offers the most direct angle of attack to the lateral recess of the fourth ventricle. However, some surgeons, particularly in Europe, have advocated for alternative approaches to ABI placement. Chief among these alternatives is the retrosigmoid or lateral suboccipital approach.[6,7]
The main argument for use of the retrosigmoid approach is the potential to preserve the cochlea and cochlear nerve if promontory testing indicates that the patient may benefit from a cochlear implant rather than an ABI.[14] Other advantages include shorter operative times and less risk of cerebrospinal fluid (CSF) leakage. Its disadvantages include difficulty in placing the lead appropriately. In one series, two of three electrodes placed via the retrosigmoid approach required revision for malpositioning. [23]
Other innovations to ABI placement include subtonsillar or telovelar approaches via a midline suboccipital craniotomy[21] and ABI placement through one of the standard approaches but with endoscope-assisted technique.[9] The advantages of a subtonsillar approach include superior visualization of the floor of the fourth ventricle and the absence of scarring of the surgical corridor. Some authors have asserted that the dorsal cochlear nucleus, which can be observed directly via a subtonsillar approach where it is found under the auditory tubercle, is a superior target for ABI placement, because the ventral cochlear nuclei are partially obscured by the cerebellar peduncle. However, the dorsal cochlear nucleus responds best to higher frequencies, including those outside the normal range used in speech.
Regardless of the suitability of the dorsal cochlear nucleus as a target for ABI, the telovelar approach, in which the inferior medullary velum is split and the taenia are divided from their attachment to the floor of the fourth ventricle, requires a midline suboccipital craniotomy or craniectomy. However, an acoustic neuroma cannot be resected via such an approach. An ABI can only be placed if the surgeon is willing to extend a retrosigmoid craniotomy inferiorly, possibly including a C1 laminectomy. Nevertheless, the telovelar or subtonsillar approach may be considered when the surgeon anticipates too much scarring or anatomical distortion related to the tumor to allow accurate placement of the electrode through either a transpetrosal or lateral suboccipital approach (i.e., when an ABI is to be placed after the operation for tumor resection).
In a cadaveric study of endoscopically assisted translabyrinthine, retrosigmoid, and even middle fossa approaches, Friedland and Wackym[9] demonstrated that visualization of the lateral recess is facilitated with the use of an endoscope. An endoscope can be wielded to excellent effect in such situations because a 30-degree angled endoscope gives the surgeon the ability to look ‘around the corner.’ Although theoretically appealing, use of an endoscope is hindered by the necessity of holding the camera and advancing the ABI paddle parallel to the scope before the device can be placed. Advances in endoscopy may increase the use of endoscopically assisted procedures.
Intraoperative Monitoring
For several reasons intraoperative monitoring of evoked auditory potentials and monitoring of the trigeminal, facial, and glossopharyngeal nerves is crucial during ABI placement.[1,10] Monitoring auditory brainstem responses can corroborate accurate electrode placement, whereas monitoring neighboring cranial nerves may detect inadvertent stimulation of regional structures. When the labyrinth has not been drilled and the cochlear nerve has been preserved, promontory testing also may be useful to predict the possible benefit of cochlear implantation.
The auditory brainstem response (ABR) is thought to reflect the summated activity of ascending portions of the auditory system. Classically, five ABR waves (i.e., waves I-V) are thought to reflect the activity of the cochlea, cochlear nerve, cochlear nuclei, olivary nuclei, and nuclei of the lateral lemniscus, respectively. In the setting of ABI placement, the cochlear or cochlear nerve waves of the ABR are not expected because the cochlear nuclear complex is stimulated directly.[24] Intraoperatively, the first wave of the ABR seen would be wave III, that of the cochlear nuclei. Ideally, three waves would be expected: those of the cochlear nuclei (III), olivary nuclei (IV), and nuclei of the lateral lemniscus (V). In practice, anywhere between one and three responses, termed P1-P3, are seen.[24]
The presence of one or more responses helps corroborate proper electrode placement. However, there are pitfalls in the interpretation of intraoperative responses, including the presence of motor contamination (i.e., motor responses transmitted from inadvertent stimulations of the trigeminal, facial, glossopharyngeal, or vagus nerves). Motor responses can be differentiated from auditory responses by both latency and amplitude. Higher amplitude waves with longer latencies are indicative of a motor response.
ABR monitoring is best understood as an adjunct to anatomical means of localizing the cochlear nuclei. Its role is simply to confirm that the ABI stimulates the auditory brainstem, with minimal or no stimulation of other structures. No correlations between the number or quality of waveform responses (i.e., P1, P2, P3) and the efficacy of ABI have been found. Moreover, as long as the surgeon is confident of adequate electrode placement based on reliable anatomical landmarks, even the complete absence of ABR waves does not necessarily predict that the ABI will fail to impart useful hearing.
Surgical Results
American Experience
The United States clinical trial of the multichannel ABI, composed of data collected from 10 domestic centers, constitutes the largest published series. It involved 92 patients receiving the Nucleus 22 (Cochlear Corporation, Englewood, CO), eight-electrode ABI, 85% of whom reported receiving auditory sensations.[8] At their six-month follow-up examination, 93% of patients receiving auditory sensations demonstrated improved lipreading, reflected in a mean improvement of 24% on the CUNY sentence test (an open-set speech comprehension test) over lipreading alone. Importantly, 85% of patients reported satisfaction with their ABI, and 74% would recommend it to others. Of patients who used the device daily (97% of those patients implanted at the second surgery (i.e., deaf patients), 65% reported using the device more than 8 hours a day.
In 13 patients the failure of the device to elicit auditory stimulation was attributed to improper electrode placement related to distorted anatomy. There were two deaths, although neither were directly attributable to the ABI. Nonauditory side effects, most commonly paresthesias involving the ipsilateral body, were common. They are attributed to stimulation of the inferior cerebellar peduncle, which lies close to the cochlear nuclei (Fig. 4).
In the House Ear Institute experience with the long-term follow-up of 71 patients receiving an eight-electrode ABI, 10 patients did not participate in follow-up.[18] Of the remaining 61 patients, 6 detected no auditory sensations after implantation. Two CSF leaks were treated by pressure bandage and lumbar drainage, and one patient developed meningitis. Eventually, 24% of the electrodes were inactivated because of nonauditory perception.
European Experience
The European experience with ABI differs from the experience in the United States in several significant ways. The latter clinical trial primarily was performed with the Nucleus 22 eight-electrode ABI while in Europe 12-, 16- and 21-electrode multichannel ABIs have been placed preferentially over eightelectrode models (including the clinical trials of Digisonix, Med-El, and the Nucleus 24 ABIs). European centers have implanted the devices for more indications than have been used in the United States. European also have used the retrosigmoid approach more often than the translabyrinthine approach.
Nevertheless, the European experience broadly mirrors the experience in the United States in terms of rates of surgical morbidity and functional outcomes. Unfortunately, heterogeneity in audiological tests performed makes direct comparison of the audiological tests impracticable. The combined results of the European Nucleus (i.e., 21-channel ABI) experience (n=58), the Digisonix clinical trial results (n=14), and the MED-EL clinical trial results (n=16) yielded 88 patients. Three patients were implanted for indications other than NF-2, including bilateral auditory neuropathy and miscellaneous tumor syndromes affecting the vestibulocochlear nerve bilaterally. Three patients died and one wound infection required explantation of the device. Of 24 patients who underwent a retrosigmoid craniotomy for electrode placement, three required revision surgery. One patient had transient but significant symptomatic cerebellar swelling. Between 86 and 100% of patients experienced auditory sensations, 63% to 100% of whom were daily users. All patients who received auditory percepts demonstrated improved speech recognition in conjunction with lipreading. A limited number of patients achieved some open-set sound-only speech recognition.
Future Directions
In the last decade ABI research has focused on increasing the number of available channels for stimulation. The ideal number of channels has not yet been established and whether more channels necessarily correlate with better chances of comprehending speech is unclear.[13] The biggest innovation in ABI design has been the recent development of penetrating electrodes for stimulation of regions of the ventral cochlear nucleus complex that have been inaccessible to surface electrodes. Such electrodes offer the potential of stimulating higher frequency cell populations and may therefore increase the number of patients receiving ABI who are able to perform pitch scaling. Even though fewer electrodes are used, such an increased range of pitch sensation might facilitate speech recognition.
Conclusions
ABIs restore meaningful hearing to patients deafened by NF-2. Almost all patients can expect to receive some auditory perceptions and awareness of environmental sounds. The ability to communicate with lipreading improves significantly in most patients, but few will achieve sufficient open-set soundonly speech comprehension to enable use of the telephone.
Appropriate expectations and motivation on the part of the patient is mandatory. Many ABI patients are initially discouraged by the quality of sound heard with an ABI. They must understand that participation in their auditory rehabilitation program is crucial to continue to improve their proficiency with the device. Such improvement can be expected to continue over many years.
References
- Brackmann DE, Hitselberger WE, Nelson RA, et al: Auditory brainstem implant: I. Issues in surgical implantation. Otolaryngol Head Neck Surg 108:624-633, 1993
- Colletti V, Carner M, Fiorino F, et al: Hearing restoration with auditory brainstem implant in three children with cochlear nerve aplasia. Otol Neurotol 23:682-693, 2002
- Colletti V, Carner M, Miorelli V, et al: Auditory brainstem implant in posttraumatic cochlear nerve avulsion. Audiol Neurootol 9:247-255, 2004
- Colletti V, Fiorino F, Carner M, et al: Auditory brainstem implantation: The University of Verona experience. Otolaryngol Head Neck Surg 127:84-96, 2002
- Colletti V, Fiorino F, Sacchetto L, et al: Hearing habilitation with auditory brainstem implantation in two children with cochlear nerve aplasia. Int J Pediatr Otorhinolaryngol 60:99-111, 2001
- Colletti V, Fiorino FG, Carner M, et al: The retrosigmoid approach for auditory brainstem implantation. Am J Otol 21:826-836, 2000
- Colletti V, Sacchetto L, Giarbini N, et al: Retrosigmoid approach for auditory brainstem implant. J Laryngol Otol 114 (Suppl 27):37-40, 2000
- Ebinger K, Otto S, Arcaroli J, et al: Multichannel auditory brainstem implant: US clinical trial results. J Laryngol Otol 114 (Suppl 27):50-53, 2000
- Friedland DR, Wackym PA: Evaluation of surgical approaches to endoscopic auditory brainstem implantation. Laryngoscope 109:175-180, 1999
- Frohne C, Matthies C, Lesinski-Schiedat A, et al: Extensive monitoring during auditory brainstem implant surgery. J Laryngol Otol 114 (Suppl 27):11-14, 2000
- Grayeli AB, Bouccara D, Kalamarides M, et al: Auditory brainstem implant in bilateral and completely ossified cochleae. Otol Neurotol 24:79-82, 2003
- Klose AK, Sollmann WP: Anatomical variations of landmarks for implantation at the cochlear nucleus. J Laryngol Otol 114 (Suppl 27):8-10, 2000
- Kuchta J, Otto SR, Shannon RV, et al: The multichannel auditory brainstem implant: How many electrodes make sense? J Neurosurg 100:16-23, 2004
- Marangos N, Stecker M, Laszig R: Topodiagnosis of deafness: Strategy for treatment of neurofibromatosis type 2. J Laryngol Otol 114 (Suppl 27):3-7, 2000
- Marangos N, Stecker M, Sollmann WP, et al: Stimulation of the cochlear nucleus with multichannel auditory brainstem implants and long-term results: Freiburg patients. J Laryngol Otol 114 (Suppl 27):27-31, 2000
- Matthies C, Thomas S, Moshrefi M, et al: Auditory brainstem implants: Current neurosurgical experiences and perspective. J Laryngol Otol 114 (Suppl 27):32-36, 2000
- Otto SR, Brackmann DE, Hitselberger W: Auditory brainstem implantation in 12- to 18-year-olds. Arch Otolaryngol Head Neck Surg 130:656-659, 2004
- Otto SR, Brackmann DE, Hitselberger WE, et al: Multichannel auditory brainstem implant: Update on performance in 61 patients. J Neurosurg 96:1063-1071, 2002
- Quester R, Schroder R: Topographic anatomy of the cochlear nuclear region at the floor of the fourth ventricle in humans. J Neurosurg 91(3): 466-476, 1999
- Quester R, Schroder R, Klug N: Optimization of microsurgical operation technique to insert auditory brainstem implants, taking into account the results of a morphometric study [in German]. HNO 52:706-713, 2004
- Seki Y, Samejima N, Kumakawa K, et al: Subtonsillar placement of auditory brainstem implant. Acta Neurochir Suppl 87:85-87, 2003
- Sollmann WP, Laszig R, Marangos N: Surgical experiences in 58 cases using the Nucleus 22 multichannel auditory brainstem implant. J Laryngol Otol 114 (Suppl 27):23-26, 2000
- Vincent C, Zini C, Gandolfi A, et al: Results of the MXM Digisonic auditory brainstem implant clinical trials in Europe. Otol Neurotol 23:56-60, 2002
- Waring MD: Auditory brain-stem responses evoked by electrical stimulation of the cochlear nucleus in human subjects. Electroencephalogr Clin Neurophysiol 96:338-347, 1995
