
Multiple Dural Arteriovenous Fistulas Presenting with Rapidly Progressive Dementia: Case Report and Review of the Literature
David J. Teeple, MD
Cameron G. McDougall, MD*
S. Gabe Rice, MS
Patricio F. Reyes, MD
Divisions of Neurology and *Neurological Surgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
Rapidly progressive dementia is usually associated with prion disease, exposure to neurotoxins, and carcinomatous meningitis. We report a 70-year-old man with relentless dementia and multiple dural fistulas who recovered from most of his neurobehavioral deficits after undergoing a two-stage embolization. We suggest that multiple dural fistulas should be considered in the differential diagnosis of patients exhibiting rapid cognitive decline.
Key Words: Dementia, dural arteriovenous fistula
Abbreviations used: CSF, cerebrospinal fluid; CT, computed tomography; DAVFs, dural arteriovenous fistulas; EEG, electroencephalography; MR, magnetic resonance; PCR, polymerase chain reaction; PET, positron emission tomography
Dementia affects 10% of persons 65 years and older and as many as 50% of those older than 85 years.4 Dementia usually refers to medical conditions characterized clinically by a gradual decline in cognition, behavior, and activities of daily living. Rapid onset dementia is less common, and its differential diagnosis includes prion disease (Creutzfeldt-Jakob disease), neoplastic processes (lymphoma, paraneoplastic syndrome), central nervous system infections (syphilis, Herpes simplex), acute thiamine deficiency, organ failure, and toxic metabolic conditions. Careful and comprehensive medical neurobehavioral assessments coupled with a thorough history, laboratory studies, and neuroimaging (CT and MR imaging) are necessary to identify the cause. In certain cases EEG and CSF testing (smear, culture, antigen and antibody levels, PCR, 14-13-3 protein) are essential in establishing the diagnosis. We report a case of rapidly progressive dementia caused by multiple DAVFs.
Case Report
A 70-year-old man with a history of hypothyroidism was transferred to our institution for evaluation of rapidly progressive dementia of an undetermined etiology. Four months earlier, the patient was first noted to have cognitive and behavioral symptoms. Before the onset of his symptoms, the patient reportedly had slept through the night in a room that had recently been fumigated. The next morning he had difficulty finding the right words, a symptom associated with agitation and diminished comprehension. He saw his primary care physician and neurologist who found mild cognitive and attentional deficits.
The patient’s neurologic evaluation included brain MR imaging with and without contrast, EEG, sedimentation rate, an antinuclear antibody test, the venereal disease research laboratory test, level of angiotensin-converting enzyme, human immunodeficiency virus testing, lumbar puncture, and thyroid function studies. MR imaging showed mild cerebral atrophy and EEG showed generalized slowing. Pertinent laboratory studies revealed slightly elevated levels of CSF protein and mild hypothyroidism. No treatment was prescribed.
In the ensuing weeks the patient’s neurobehavioral status declined rapidly. He became increasingly confused, disoriented, and nonverbal. He was unable to recognize his wife, friends, and relatives and became completely dependent on his wife for his activities of daily living. A month later the patient was prescribed steroids that mildly improved his cognitive functioning for a few days. However, the patient’s condition continued to decline, and he was transferred to our neurology service for further evaluation.
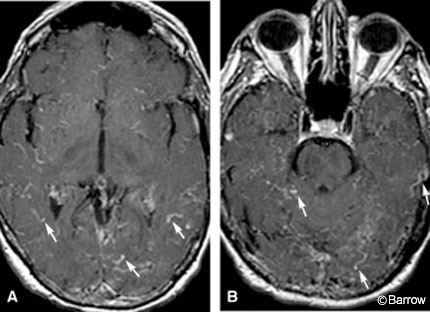
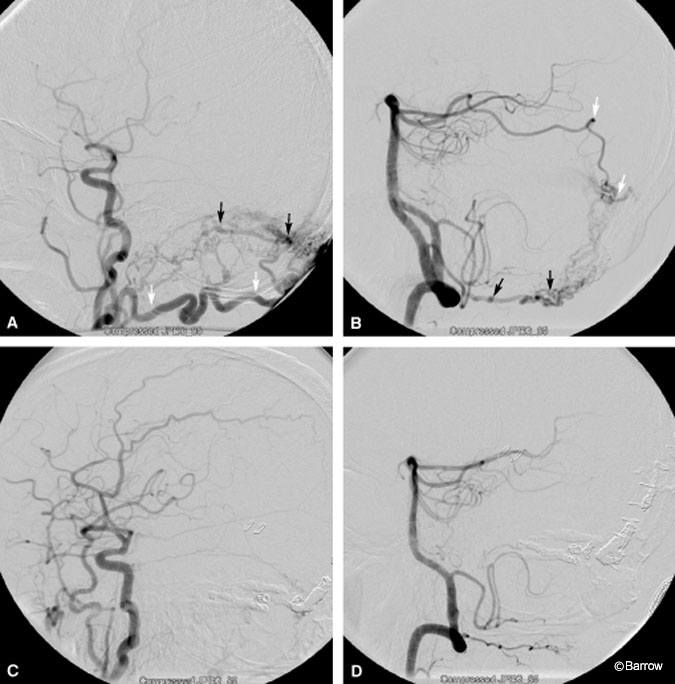
On admission the patient exhibited global language deficits, an inability to follow commands, fluctuating level of consciousness, and startle myoclonus. Brain MR imaging with and without contrast showed prominent cortical vessels (Fig. 1). Four-vessel cerebral angiography showed four DAVFs involving the transverse sinuses bilaterally, the superior sagittal sinus, and torcula (Fig. 2).
The patient underwent endovascular surgery to obliterate the DAFVs, which was performed successfully in a two-staged embolization. His postoperative course was uneventful, and his neurologic deficits gradually improved. He underwent aggressive rehabilitation for 2 weeks and was conversant and ambulatory with minimal assistance at discharge.
The patient returned to the clinic for follow-up examinations 1 month, 3 months, and 5 months after surgery. At his 3-month follow-up examination, he began to exhibit mild confusion, word-finding difficulty, and memory impairment for recent events. A second cerebral angiogram showed a previously unnoticed fistula that required treatment via an open microsurgical approach. Postprocedural angiography confirmed complete obliteration of the fistula (Fig. 2). Since the operation, the patient’s cognitive function has continued to improve. He has only mild deficits in short-term memory.
Discussion
Approximately 10 to 15% of all intracranial arteriovenous malformations consist of adult-type DAVFs.[18] The peak incidence of DAVFs is between the fourth and sixth decades, but they can occur at any time. DAVFs are usually considered to be acquired lesions. Trauma is a well-known cause of acute onset DAVF attributed to damage of nearby dural arteries and veins. DAVFs also have been reported subacutely after trauma, infection, and surgery. Our patient had no history of trauma, infection, or surgery before his neurologic symptoms appeared. Thrombosis of a vein is also a common feature of DAVFs.
Although the mechanism underlying the formation of fistulas is not yet understood, there are two hypotheses. Fistulas are thought to develop when normal physiologic pathways become pathologic arteriovenous shunts, or they are attributed to the release of other angiogenic factors that cause the shunts to develop.[7]
Common signs and symptoms of DAVFs include headache, bruit, pulsatile tinnitus, stroke-like symptoms, and hemorrhage.[5,15] Various mechanisms, including arterial steal, high venous flow, venous thrombosis, venous ischemia, and venous rupture, have been proposed to account for the sequelae of DAVF.[15] The differentiation of high- and low-risk DAVFs is based on venous drainage patterns: Increased cortical venous reflux is consistent with a more aggressive course.[1,2]
A less clearly understood problem is cognitive impairment as a result of a DAVF. Obrador et al.[16] reviewed 96 cases of DAVFs and found that alteration of mental status was a prominent symptom in 12% of their patients. In a review of 45 published cases of DAVFs involving the transverse and sigmoid sinuses, Ishii et al.[10] identified dementia in 22% of the patients. Hurst et al. found that 12.5% of 40 consecutive patients with DAVFs had cognitive dysfunction as their presenting feature.[8] The MR imaging findings of these patients consistently showed T2-weighted hyperintensity and enlarged cortical vessels. Delayed venous emptying was a common finding on digital subtraction angiography. The findings on MR imaging and angiography are consistent with a state of venous hypertensive encephalopathy, and it is the likely cause of these symptoms.[6,8,11,13,14]
Of 10 patients with a DAVF studied with PET, the primary clinical manifestation in four was significant cognitive deterioration, ranging from a disturbance in consciousness to memory problems. PET showed decreased regional blood flow; increased oxygen extraction; and increased cerebral blood volume in the entire cerebrum in two patients, in the thalamus bilaterally in one patient, and in the left temporal lobe in one patient.12 These findings suggest that ischemia, possibly related to venous hypertension involving either the entire cerebrum or the thalamus, could lead to symptoms of altered cognition and behavior. In multiple other studies, cognitive function has improved after treatment of fistulas.[3,9,11,17]
Conclusion
Many causes of rapid onset dementia, such as prion disease, may be devastating and irreversible. It is critical to consider reversible etiologies to establish an early diagnosis and to deliver prompt and definitive management. This case illustrates a treatable cause of rapidly progressive dementia as well as the need to consider multiple DAVFs in the differential diagnosis of dementia.
References
- Borden JA, Wu JK, Shucart WA: A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 82:166-179, 1995
- Cognard C, Gobin YP, Pierot L, et al: Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology 194:671-680, 1995
- Datta NN, Rehman SU, Kwok JC, et al: Reversible dementia due to dural arteriovenous fistula: A simple surgical option. Neurosurg Rev 21:174-176, 1998
- Evans DA, Funkenstein HH, Albert MS, et al: Prevalence of Alzheimer’s disease in a community population of older persons. Higher than previously reported. JAMA 262:2551-2556, 1989
- Festa JR, Lazar RM, Marshall RS, et al: Dural arteriovenous fistula presents like an ischemic stroke. Cogn Behav Neurol 17:50-53, 2004
- Greenough GP, Mamourian A, Harbaugh RE: Venous hypertension associated with a posterior fossa dural arteriovenous fistula: Another cause of bithalamic lesions on MR images. AJNR Am J Neuroradiol 20:145-147, 1999
- Hamada Y, Goto K, Inoue T, et al: Histopathological aspects of dural arteriovenous fistulas in the transverse-sigmoid sinus region in nine patients. Neurosurgery 40:452-456, 1997
- Hurst RW, Bagley LJ, Galetta S, et al: Dementia resulting from dural arteriovenous fistulas: The pathologic findings of venous hypertensive encephalopathy. AJNR Am J Neuroradiol 19:1267-1273, 1998
- Hurst RW, Hackney DB, Goldberg HI, et al: Reversible arteriovenous malformation-induced venous hypertension as a cause of neurological deficits. Neurosurgery 30:422-425, 1992
- Ishii K, Goto K, Ihara K, et al: High-risk dural arteriovenous fistulae of the transverse and sigmoid sinuses. AJNR Am J Neuroradiol 8:1113-1120, 1987
- Ito M, Sonokawa T, Mishina H, et al: Reversible dural arteriovenous malformation-induced venous ischemia as a cause of dementia: Treatment by surgical occlusion of draining dural sinus: Case report. Neurosurgery 37:1187-1191, 1995
- Iwama T, Hashimoto N, Takagi Y, et al: Hemodynamic and metabolic disturbances in patients with intracranial dural arteriovenous fistulas: Positron emission tomography evaluation before and after treatment. J Neurosurg 86:806-811, 1997
- Kataoka H, Miyamoto S, Nagata I, et al: Venous congestion is a major cause of neurological deterioration in spinal arteriovenous malformations. Neurosurgery 48:1224-1229, 2001
- Kurata A, Miyasaka Y, Yoshida T, et al: Venous ischemia caused by dural arteriovenous malformation. Case report. J Neurosurg 80:552-555, 1994
- Lasjaunias P, Chiu M, ter Brugge K, et al: Neurological manifestations of intracranial dural arteriovenous malformations. J Neurosurg 64:724-730, 1986
- Obrador S, Soto M, Silvela J: Clinical syndromes of arteriovenous malformations of the transverse-sigmoid sinus. J Neurol Neurosurg Psychiatry 38:436-451, 1975
- Uchino A, Numaguchi Y, Holloway RG, et al: Reversible symptomatic venous congestion after treatment of dural arteriovenous fistula using NBCA. Radiat Med 16:477-481, 1998
- Vinuela F, Fox AJ, Pelz DM, et al: Unusual clinical manifestations of dural arteriovenous malformations. J Neurosurg 64:554-558, 1986
