Find a Clinical Trial
Improvements in medicine wouldn’t be possible without clinical trials. These voluntary research studies allow us to carefully evaluate the safety and effectiveness of new medical and surgical treatments, preventative measures, and diagnostic tests.
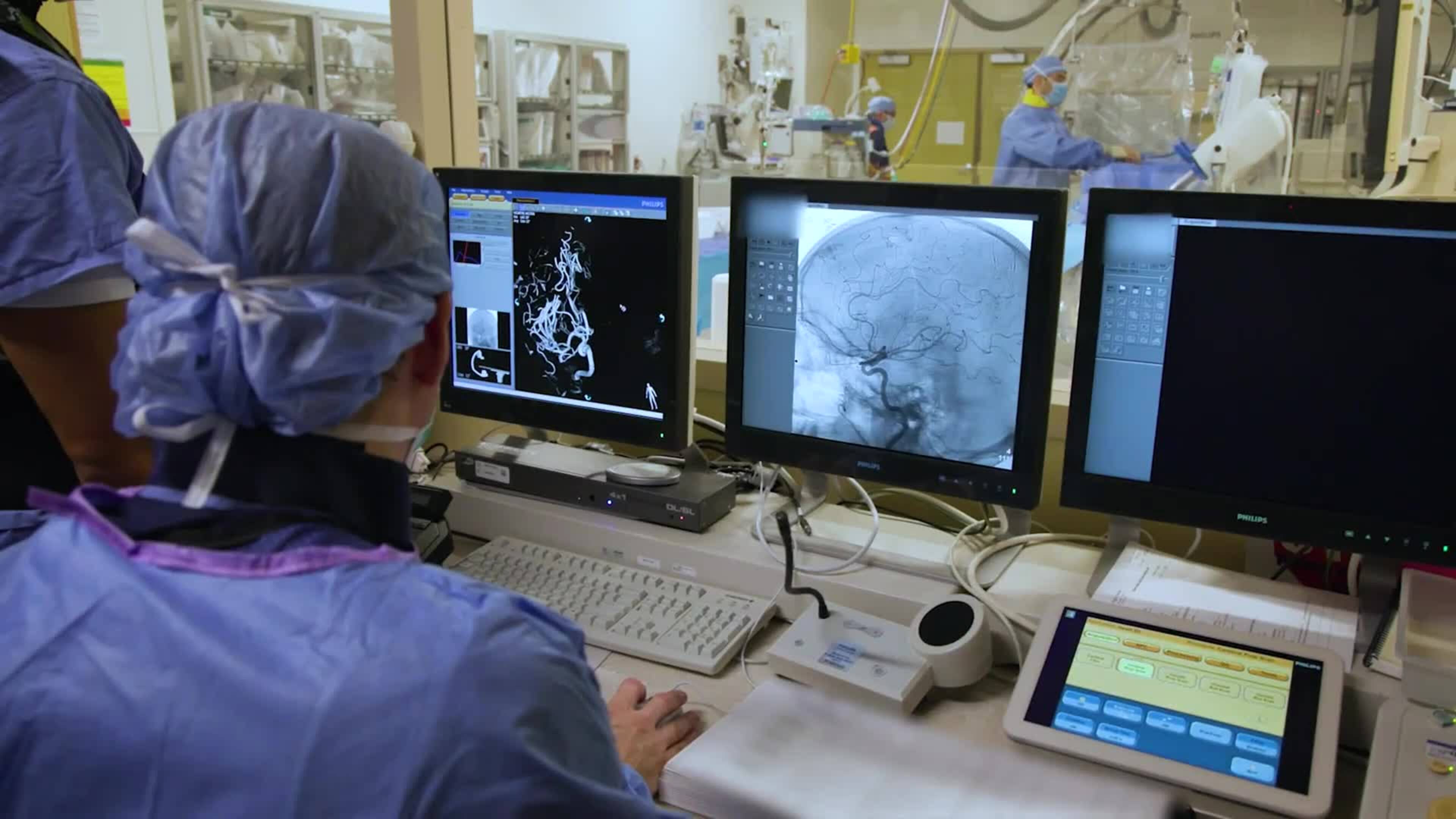
At Barrow Neurological Institute, innovation and discovery are deeply ingrained in our history and vital to our mission. From refining the cardiac standstill procedure to exploring deep brain stimulation as a therapy for Alzheimer’s disease, our experts work tirelessly to care for our patients today while working to find the treatments of tomorrow.
Clinical trial participants have the opportunity to help advance scientific knowledge. They also have the potential to receive the newest treatment for their diagnosis. Our goal is to be able to offer every patient the option to participate in a clinical trial. We have more than 300 active clinical trials in various phases for many different neurological conditions—both common and rare. Additionally, we have several dozen full-time employees dedicated to clinical research.
Barrow is also home to the Ivy Brain Tumor Center, which opened in 2018. It is the largest Phase 0 clinical trials program in the world and the first of its kind for neuro-oncology. The Phase 0 approach allows us to tailor treatment to each individual tumor and rapidly measure its effects. This provides hope for patients with the most aggressive brain tumors, which may not respond to traditional treatments.
Our clinical research space demonstrates the Barrow creed to accept challenges, reject norms, and push the boundaries of neuroscience.
Interested in participating in a clinical trial? Browse our database to learn more about current active trials for your diagnosis.
What is Informed Consent?
Informed consent means that as a research volunteer, you are given all the information available about a clinical trial so that you understand what is involved. It’s more than just a signature on a form. It’s the process of information exchange that may include subject recruitment materials, verbal instructions, question and answer sessions, and measures of subject understanding.
Your physician or nurse should explain the clinical trial to you, including any risks. Prior to participating in the study, you will be given an informed consent form. Be certain to read and consider it carefully. If something in the form is not clear to you, ask your physician or nurse to explain it to you. The informed consent should include the following:
- The reason for the trial.
- The treatment procedures and schedule.
- Any potential risks or benefits.
- Alternatives to participating in the trial.
- An explanation of your rights as a research volunteer.
It’s up to you to decide whether or not you want to take part in a clinical trial. If you decide to take part in the study, you will sign the consent form. Once you sign the form, you will be given a copy for your records. Please note that the informed consent contains important information you may refer to during the trial. If you decide you do not want to participate, you have the option to decline. If you choose not to participate in the trial, please know that your care will not be affected in any way.
There are four phases of a clinical trial:
Phase I: In this phase, it is usually the first time a new treatment, drug or device is used in humans. The treatment under investigation is given to a small number of research volunteers at a very low dose or setting, and is increased over time according to the response. The purpose of this phase is to determine the best way to give the new treatment – either by mouth, injected into the blood or injected into the muscle – and how much of it can be given safely.
Phase II: Phase II helps to determine the effect of a research treatment on a particular disease or condition. Phase II trials involve the development of a protocol, or plan, about how the trial will be conducted and how the data will be analyzed. These plans are submitted to the Institutional Review Board (IRB), who must approve the protocol before the clinical trial begins. The IRB is a group of health professionals and non-medical persons whose job it is to review and monitor clinical research to protect the safety and rights of participants. Some research is also reviewed and monitored by federal agencies, such as the National Institutes of Health or the Food and Drug Administration. In a Phase II clinical trial, there may or may not be a control group. Control groups receive standard, current care. Treatment groups receive the experimental therapy.
Phase III: This phase compares the new treatment with the standard treatment on a larger group of research volunteers to determine effectiveness, dosing, and optimal characteristics of a treatment, drug or device. The same review and approval process takes place as in a Phase II clinical trial. In this phase, there is most likely a control group and a treatment group. Research volunteers are randomized, or assigned to a group based on chance.
Phase IV: This phase of research is conducted after approval has been given by the Food and Drug Administration. The purpose of a Phase IV clinical trial is to continue testing the study drug or treatment to collect information about its side effects in various populations and any side effects associated with long-term use.
When you’re considering participating in a clinical trial, you need to know as much information about the study and its components as possible. We’ve included a few of the questions you might ask before becoming a research volunteer:
- What is the purpose of the study?
- How long will the trial last?
- What other options or choices do I have if I decide not to participate?
- What tests and treatments does the study involve?
- What are the short- and long-term side effects risks and benefits of this trial?
- What are the possible side effects?
- How do these compare to my current treatments?
- What type of long-term follow up care is part of the study?
- Who will pay for the treatment and all other expenses related to the study?
- How can I end my participation in the study if I change my mind?
- Whom do I contact for questions and information about the study?
- The patient has the right to considerate and respectful care.
- The patient has the right to know, by name, the physician responsible for coordinating his or her care.
- The patient has the right to obtain from his or her physician complete current information about diagnosis, treatment, and prognosis in easily understandable terms. If it is medically inadvisable to give such information to the patients, it will be given to a legally authorized representative.
- The patient has the right to receive from his or her physician information necessary to give informed consent prior to the start of any procedure or treatment. Except in emergencies this will include, but not necessarily be limited to, a description of the specific procedure or treatment, any risks involved, and the probable duration of any incapacitation. When there are alternatives to therapeutically designed research protocols, the patient has the right to know about them. The patient also has the right to know the name of the person responsible for directing the procedures or treatment.
- The patient has the right to refuse to participate in research, to refuse treatment to the extent permitted by law, and has the right to be informed of the medical consequences of these actions including possible dismissal from the study and discharge from the institution. If discharge would jeopardize the patient’s health, he or she has the right to remain under the hospital’s care until discharge or transfer is medically advisable.
- The patient has the right to be transferred to another facility when his or her participation in the study is terminated, providing the transfer is medically permissible, the patient has been informed of the needs for and alternatives to such a transfer, and the facility has agreed to accept the patient.
- The patient has the right to privacy concerning the medical care program. Case discussion, consultation, examination, and treatment are confidential and will be conducted discreetly. The patient has the right to expect that all communications and records pertaining to care will be treated as confidential to the extent permitted by law.
- The patient has the right to routine services whenever hospitalized in connection with the active protocol for which he or she is eligible; these services will generally include diagnostic procedures and medical treatment deemed necessary and advisable by the professional staff. Complicating chronic conditions will be noted, reported to the patient, and treated as necessary without the assumption of long-term responsibility for their management. The patient may be returned for long-term or definitive care of these conditions to the referring physician or to other appropriate medical resources.
- The patient has the right to expect that medical information about him or her discovered at the hospital, as well as an account of his or her medical program here, will be communicated to the referring physician.
- The patient has the right, at any time during the medical program, to designate additional physicians or organizations to receive medical updates. The patient should inform the Outpatient Department staff of these additions.
- The patient has the right to know in advance what appointment times and physicians are available and where to go for continuity of care provided by the hospital when such care is required under the study for which the patient was admitted.
For more information, please visit the National Institutes of Health site.