
Hypoglossal Nerve Stimulation Implant
Overview
The hypoglossal nerve stimulation implant is a treatment for obstructive sleep apnea in people who don’t tolerate continuous positive airway pressure (CPAP) therapy. The implantable device helps keep the airway open by stimulating the hypoglossal nerve, which controls the muscles that move your tongue.
The hypoglossal nerve stimulation implant treats moderate to severe obstructive sleep apnea, the most common type. In sleep apnea, breathing stops and starts repeatedly during sleep. These pauses can last from a few seconds to a few minutes, leading to low oxygen levels and disrupted sleep. Sleep apnea can sound like loud snoring, gasping, or choking during sleep. It can also cause daytime sleepiness, difficulty concentrating, and irritability.
An estimated 30 million people in the U.S. struggle with sleep apnea–almost nine percent of all Americans. Still, only around six million individuals have been diagnosed with the condition.
Did you know?
The name hypoglossal is derived from the Greek word hypo, meaning under, and glossal, which means tongue. The job of the hypoglossal nerve is to carry signals to and from your brain to control muscle movement under your tongue.
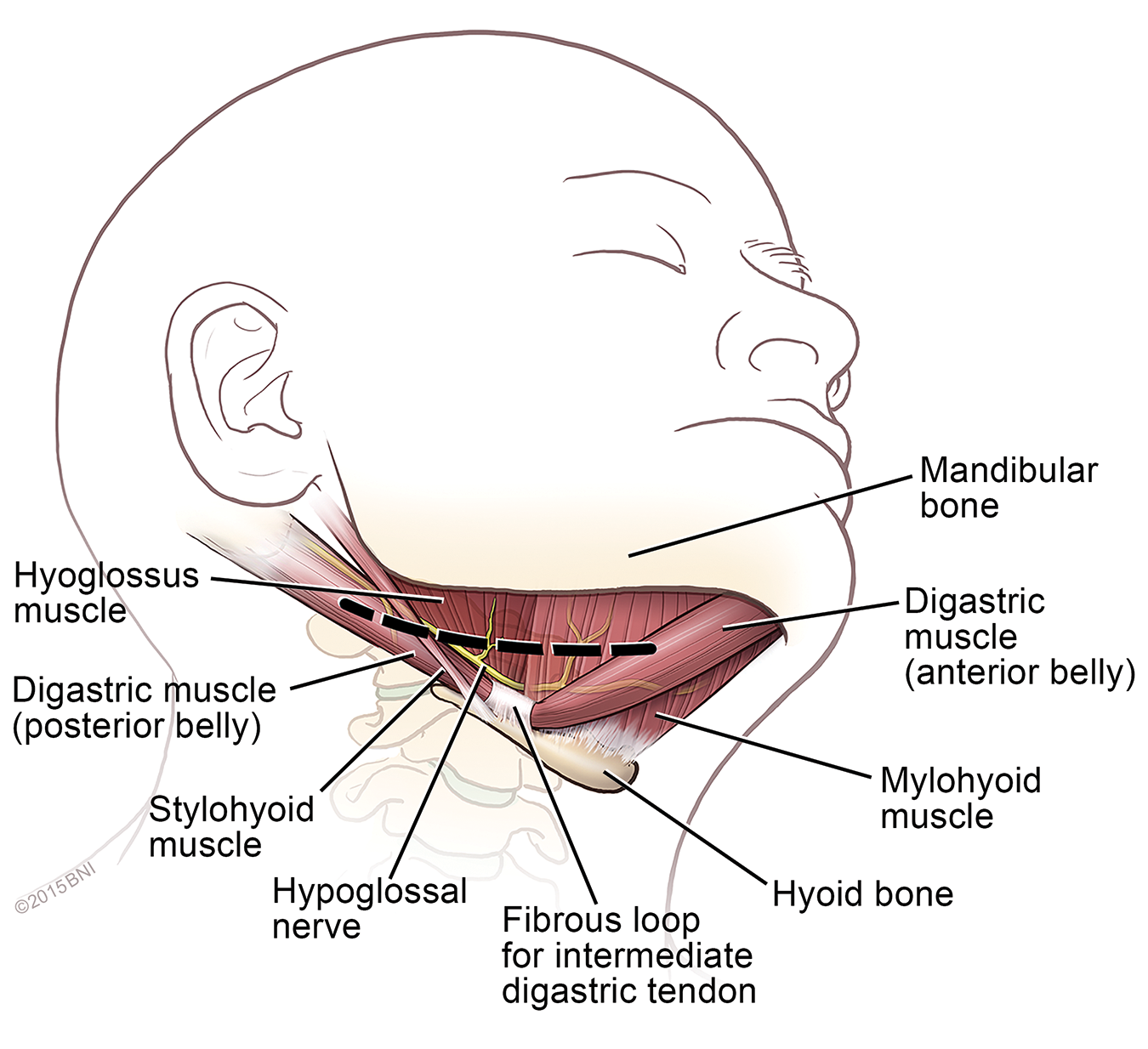
How It Works
The hypoglossal nerve stimulator is made up of three essential components and includes:
- A stimulation lead: The stimulation lead features a cuff that wraps around a branch of the hypoglossal nerve under the tongue. This electrode stimulates the nerve, causing the tongue and other airway muscles to move forward, opening the airway and allowing for a full breath.
- A sensing lead: This small, thin wire has a sensor at the end that’s placed next to the ribs to detect when a breath is being taken.
- A pulse generator: Much like a pacemaker, this small, flat device is implanted under the skin in the chest, right below the collarbone. The sensing lead sends a signal to the pulse generator when a breath is taken; then, the pulse generator sends a signal to the hypoglossal nerve to open the airway during that breath.
Steps of Surgery
Hypoglossal nerve stimulation implant surgery takes between two to three hours and is done under general anesthesia.
The procedure follows these general steps:
- First, your surgeon will make a small incision under your jaw to reach the hypoglossal nerve.
- Then, the electrode is placed around the nerve, using sutures to secure it.
- Next, your surgeon makes a second incision in your chest, placing the sensing lead between your ribs.
- A small tunnel is made under the skin to connect the two incisions. The pulse generator is placed in the chest incision, and all the wires are hooked up.
- Your surgeon tests the connection, confirming that each of the three components functions correctly.
- Finally, the incisions are closed by your surgeon.
As an outpatient procedure, you or your loved one will go home the same day as the surgery. Most patients recover within a week, experiencing only mild discomfort.
As with any surgery, there’s a small risk of infection at the incision sites. Temporary pain, tenderness, bruising, or swelling of the chest or neck can occur after surgery, as well as minor surgical scarring. The leads or electrodes can shift in rare cases, requiring device adjustments or revision surgery. There’s also a rare risk of injury to the hypoglossal nerve, leading to tongue weakness or difficulty moving the tongue.
Your hypoglossal nerve stimulation implant will be activated about four weeks post-surgery to ensure a full recovery. Once activated, you turn on the device using a remote control before you go to sleep—and there’s even a “delay feature” that allows you to set the start of the device after you fall asleep. Upon waking, you’ll turn it off, like you would for an alarm or sound machine.
To determine how well your implant works, a sleep study will need to be done to assess the device’s effectiveness and make necessary adjustments to the stimulation settings. This sleep study is typically completed three to six months after your surgery. Your provider may adjust the device once or twice yearly to ensure the implant works as intended. The hypoglossal nerve stimulation implant’s battery typically lasts 10 to 12 years and requires minor surgery to replace it if needed.
Common Questions
Am I a good candidate for the hypoglossal nerve stimulation implant?
The hypoglossal nerve stimulation implant has strict eligibility criteria. Typically, it’s approved for patients with moderate-to-severe obstructive sleep apnea who:
- Have tried using a CPAP machine but did not see sleep improvements;
- Have a BMI under 40, although some providers may require a BMI under 35;
- Are healthy enough to tolerate a two-hour surgery
- Have an airway anatomy suitable for stimulation. This is evaluated during another procedure called a drug-induced sleep endoscopy (DISE)
What kind of results can I expect with the hypoglossal nerve stimulation implant?
Studies using the Apnea-Hypopnea Index (AHI) show a 50 to 80 percent decrease in apnea events per hour. The list of expected results can include:
- Improved oxygen levels: Overall oxygen levels improve during sleep as oxygen desaturation events decrease.
- Better sleep: Most hypoglossal nerve stimulation implant patients report fewer awakenings and deeper, more restful sleep following surgery and device activation.
- Increase in daytime alertness: Due to reduced sleep disruptions, patients will experience less daytime sleepiness, reporting better focus and improved moods.
- Significant reduction in snoring: Many patients and their bed partners report a dramatic decrease—or complete elimination—of snoring.
Most hypoglossal nerve stimulation implant patients experience a life-improving change in their sleep quality, energy levels, and well-being. Between 80 and 90 percent of patients report a significant improvement in their moderate to severe sleep apnea symptoms, and upwards of 90 percent continue using the device long-term.
Are there side effects from the hypoglossal nerve stimulation implant?
While the hypoglossal nerve stimulation implant is generally safe and effective, there can be some side effects after your outpatient surgery, such as:
- Tongue discomfort or soreness: Due to the new nerve stimulation, some individuals may experience mild tingling, twitching, or soreness in the tongue.
- Dry mouth: Since the tongue is slightly repositioned, mouth breathing can increase, leading to mouth dryness.
- Swallowing difficulty: Mild swallowing issues may occur, mainly when the device is active at night.
- Mild speech changes: There may be some difficulty pronouncing certain words or slight slurring, especially in the first few weeks after surgery.
Most side effects are mild and temporary, and your team can adjust your device’s stimulation settings to improve your overall comfort.
Can I have an MRI if I have the hypoglossal nerve stimulation implant?
If you require a magnetic resonance imaging (MRI) scan, it’s important to let your technologist know you have the hypoglossal nerve stimulation implant so that they can have all the necessary information to proceed or seek an alternate option. With some of the newer implant models, an MRI can be done with precautions.
Can the hypoglossal nerve stimulation implant be removed?
Yes, the hypoglossal nerve stimulation implant can be removed if needed. While it’s designed to be a permanent solution for moderate-to-severe obstructive sleep apnea, some patients may need or choose to have it removed due to the following:
- Ineffectiveness or side effects: Your surgeon can remove the implant if it doesn’t sufficiently reduce your sleep apnea symptoms or if side effects like tongue discomfort or swallowing issues persist.
- Infection or Complications: In rare cases, infection at the implant site or device malfunction may necessitate implant removal.
- Lifestyle changes: The implant can be removed if you experience significant weight loss or have another treatment that eliminates the need for it.
- Patient preference: You may decide you no longer want the device.
Removal of the hypoglossal nerve stimulation implant device is also performed as an outpatient surgery, similar to the implant procedure.
That said, for many individuals who cannot tolerate CPAP therapy, the benefits of hypoglossal nerve stimulation implant surgery outweigh the risks. Tens of thousands of patients worldwide have received the implant, and that number increases each year.
The most widely used hypoglossal nerve stimulator, the Inspire device, has been FDA-approved since 2014, and an increasing number of insurance providers now cover the procedure, making it a more accessible option.



