
Bone Graft Harvest
Authors
Robert M. Galler, DO*
Volker K. H. Sonntag, MD
Division of Neurological Surgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
*Current address:
Department of Neurological Surgery, State University of New York, Stony Brook, NY
Abstract
Spinal fusion with or without instrumentation requires the use of bone graft. Bone graft may be autogenous or exogenous. It may be acquired from various sites and exists in various forms. The methods of obtaining bone graft as well as the properties of each type are reviewed.
Key Words: bone graft, fusion, spine
The use of transplanted bone for surgical fusion of the spine is a well-established technique. Since first reported in 1911,[1] different methods for acquiring and using bone graft have been developed. The most popular sites for harvesting bone grafts are the region of the spine that has been decompressed, the rib, and the iliac crest.
The ideal bone graft has specific features that allow healing and eventual fusion at the operative site: osteogenesis, osteoconduction, and osteoinduction. Osteogenesis refers to the native capacity to form bone. This process is accomplished by osteoblasts under the influence of many factors such as bone morphogenetic protein (BMP). Cells capable of this task are found in living marrow. Allograft lacks this capacity because the marrow is absent. The influence exerted on these cells is called osteoinduction. Methods of producing agents that effectively induce bone formation, such as BMP, have been the focus of extensive research and are covered fully elsewhere. Osteoconduction is the physical property present in the structural composition of a bone graft that acts as a scaffold for new bone to form. Allograft and autograft can both accomplish this goal. Allograft acts purely as a osteoconductive material while autograft has all three features.[8] Although autograft has a biological advantage, it requires more effort to acquire and its harvest is associated with potential complications. Postoperative pain, wound infection, numbness, and cosmetic deformity can occur at the graft site.[10]
Types of Bone Graft
Cancellous Graft
Cancellous bone graft from an autologous source is an excellent medium for fusion. It has all three properties of an ideal graft and is readily available. When obtained for fusion of the cervical spine, purely cancellous bone is most often used to pack a strut graft or as onlay material for posterolateral arthrodesis. Cancellous bone alone is unable to withstand compressive forces and should not be used as a structural element in a surgical construct.
Cortical Grafts
Pure cortical grafts are used when structural integrity is the paramount concern. They are most often used as strut grafts in vertebral reconstructions in patients who have undergone corpectomy. These struts tend to be allograft bone in the form of fibula. The primary purpose of the strut is support and osteoconduction. The center of the graft should be filled with cancellous bone to add osteogenic and inductive properties to the graft.
Corticocancellous Grafts
The combination of the structural integrity of cortical bone and the osteogenic and inductive features of cancellous bone make corticocancellous grafts a popular choice. This type of graft is most often harvested from the iliac crest because the rib provides inadequate structural support for large reconstructions. Tricortical iliac crest graft has been used extensively for cervical spine arthrodesis for more than 50 years. Harvesting large iliac crest struts is associated with potential morbidity. Furthermore, the morphology of the iliac crest and the area of the spine being reconstructed limit their use.
Anterior Approach
Vertebral Body
Bone graft material can be obtained at the time of anterior decompression. The drilled bone that remains after the endplates are prepared should not be wasted. This material is easily collected for later use with a curette or a Penfield No. 1 dissector. The center of a strut or allograft ring can then be filled with this bone graft without the need for a separate incision.
Anterior Approach
Vertebral Body
Bone graft material can be obtained at the time of anterior decompression. The drilled bone that remains after the endplates are prepared should not be wasted. This material is easily collected for later use with a curette or a Penfield No. 1 dissector. The center of a strut or allograft ring can then be filled with this bone graft without the need for a separate incision.
Iliac Crest
The anterior approach to the iliac crest is used for anterior reconstructive procedures (Fig. 1). Cancellous or corticocancellous grafts can be obtained with this method. The patient should be positioned supine with a sandbag under the ipsilateral gluteal region to accentuate the anterior superior iliac spine. The incision is made parallel to the hip so a wide area should be cleaned and prepared. At least 2 cm of the anterior superior iliac spine needs to be kept intact to avoid injury to the insertion of the sartorius muscle and to the inguinal ligament. The lateral femoral cutaneous nerve may have an anomalous course in this region and should be avoided. The integrity of the anterior superior iliac spine should not be compromised, or a stress fracture can result from the forces of the sartorius and rectus femoris musculature.
Iliac Crest
The anterior approach to the iliac crest is used for anterior reconstructive procedures (Fig. 1). Cancellous or corticocancellous grafts can be obtained with this method. The patient should be positioned supine with a sandbag under the ipsilateral gluteal region to accentuate the anterior superior iliac spine. The incision is made parallel to the hip so a wide area should be cleaned and prepared. At least 2 cm of the anterior superior iliac spine needs to be kept intact to avoid injury to the insertion of the sartorius muscle and to the inguinal ligament. The lateral femoral cutaneous nerve may have an anomalous course in this region and should be avoided. The integrity of the anterior superior iliac spine should not be compromised, or a stress fracture can result from the forces of the sartorius and rectus femoris musculature.
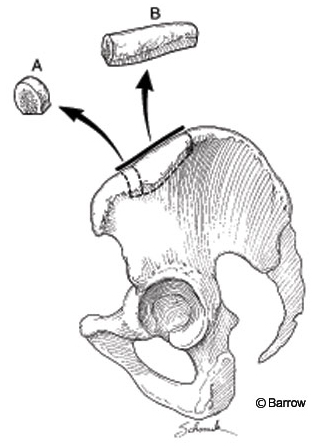
The skin is incised and dissection proceeds to the fascia overlying the iliac crest. The fascia should be opened carefully to ensure complete closure at the end of the procedure. When cancellous bone is harvested, a chisel or bone-cutting rongeur can be used to perforate the outer cortex. Once the cortex is opened, curettes or gouges are used to scrape the inner graft material. Tricortical grafts require more dissection. Electrocauterization and Cobb elevators are used to perform a subperiosteal dissection of the musculature; the peritoneal cavity on the medial side should not be violated. Careless dissection of the iliacus can injure the iliohypogastric or ilioinguinal nerves as they course over the muscle (Fig. 2). Once the iliac crest is fully exposed, the size of the graft should be measured carefully in all three dimensions. The graft is then fashioned with a reciprocating sagittal saw. Final removal of the bone may require the use of gouges and chisels to free the bone from attachments in the inferomedial region. Hemostasis of the exposed bony surfaces is achieved using Gelfoam, bone wax, or both. If necessary, a drain may be left in place overnight to avoid formation of a seroma.
Reconstruction
Much of the morbidity associated with the use of autograft has been attributed to pain at the site of harvest. This pain may be the result of injury to the nerves in the area, inadequate bony healing leading to fracture, or poor repair of the muscular attachments to the iliac crest.[3] Recently, strategies to minimize graft-related complications have been under investigation. The graft site can be reconstructed by several different methods.[2,6,7,11]
For anterior graft sites that have had only cancellous bone removed, the empty cavity of the iliac crest can be filled with allograft cancellous expander. This choice may promote internal healing and may help the crest to regain a more normal density. The defect left after a strut is removed is a more difficult problem. A large defect can leave a painful and unsightly deformity in the hip.
Such deformities can be addressed with a variety of reconstructive techniques. The gap can be filled with polymethylmethacrylate or other ceramic material,[2] which is molded to recreate the anatomy of the iliac crest. Recent approaches combine absorbable mesh with bone cement to remodel the iliac crest. This material provides a lattice against which new bone may form. The bioabsorbable nature of the mesh obviates concern about implanting another foreign body. Preliminary results using this technique have been promising.[11] However, with the advent of osteoinductive materials like BMP, the need for iliac crest reconstruction techniques may be short lived.
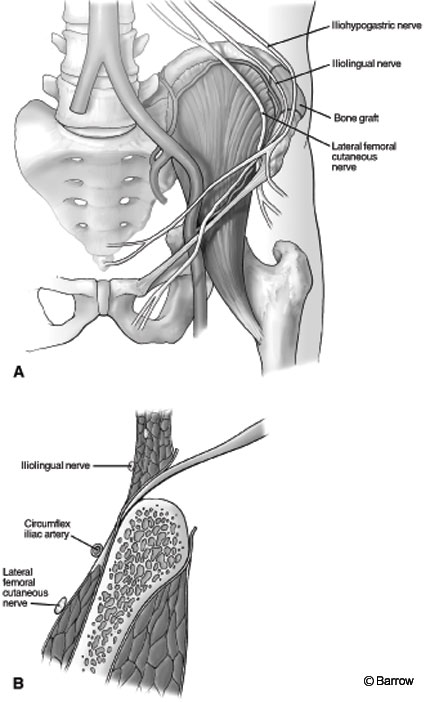
Figure 2. (A) Anterior view of graft site avoiding damage to the ilioinguinal, iliohypogastric, and lateral femoral cutaneous nerves. (B) Coronal view of dissection, which should remain in a subperiosteal plane. Cauterization should be avoided along the medial surface of the ilium to prevent injury to the ilioinguinal, iliohypogastric,and lateral femoral cutaneous nerves.
Fibular Strut Graft
Autogenous fibula may be associated with significant morbidity, but it also has been associated with high rates of arthrodesis.[5,9] In cases of failed fusion, vascularized graft can be used.[6] The patient can be placed prone, lateral, or supine. When the supine position is used, a bolster should be placed under the knee of the donor leg at midcalf so that the foot can rest in a position that keeps the leg flexed at the knee and internally rotated at the hip. The midportion of the fibula should be adequate for use in the spine. The proximal head of the fibula should be avoided to preserve function of the peroneal nerve. To preserve ankle stability, the lower 7 cm should be preserved. As much as 18 cm can be harvested for use as multiple struts.
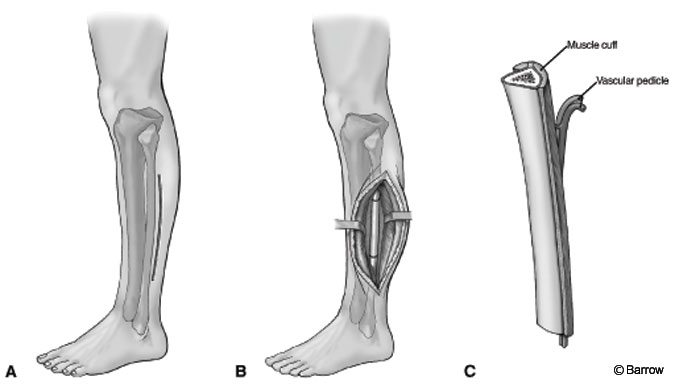
An incision is made on the lateral surface of the leg between the peroneal and soleus muscles. The plane is dissected sharply until the bone can be palpated. The fibular surface is covered in muscle origins that can be dissected free with electrocauterization using a subperiosteal technique. For vascularized fibular grafts, a muscular cuff that contains the nutrient vessels is preserved upon removal. A vascular pedicle is used for possible anastomosis. A curved elevator is used to free the deep surface. The bone can be cut with a saw while exerting care to preserve the surrounding musculature. Meticulous hemostasis is then achieved (Fig. 3). The fascia is not closed to avoid the possibility of compartment syndrome, and a drain is placed. The fibula carries as much as 15% of the axial load and is the origin of many muscles of the leg. Consequently, walking may be uncomfortable for as long as 9 months after surgery. In children, radiographic studies of the leg should be performed periodically to assess for the development of valgus tilt of the ankle.[4]
Posterior Approach
Spinous Process and Lamina
Saving the posterior elements removed during laminectomy is the simplest method of obtaining bone graft material. This region primarily consists of cortical bone but does have elements of cancellous material throughout. This graft is best utilized by carefully separating it from all soft tissues and grinding it into a heavy paste. When collected appropriately, this material may be enough to avoid a second incision.
Rib
Rib autograft may be used to supplement spinal fusion. It is corticocancellous but lacks the structural integrity of iliac crest. It is somewhat flexible and can be contoured to accommodate the posterolateral cervical spine.
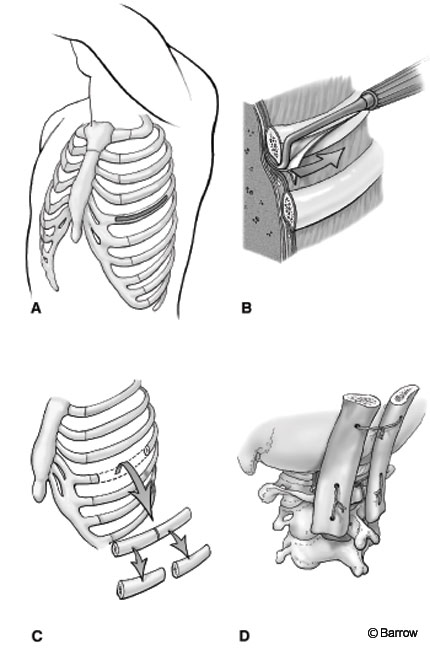
An incision is made over the rib to be removed. The tissues are dissected free with electrocauterization from the articulation to the measured length needed. A subperiosteal dissection is performed to expose the bare surface of the rib. Care should be taken near the inferior aspect of the rib to avoid the neurovascular bundle. Once the superficial area of bone has been exposed, a periosteal Doyen elevator is used to dissect the soft tissues circumferentially from the rib. This dissection should be performed carefully to avoid entering the pleural space. A rib cutter may be used to incise the rib so that it can be removed. An oscillating saw or high-speed drill is preferred because a rib cutter tends to splinter the rib adjacent to the cut surfaces (Fig. 4). The parietal pleura should be irrigated and inspected for an air leak. Small defects in the pleura can be repaired primarily. The wound is closed in layers. Postoperatively, a chest radiograph is obtained to monitor for pneumothorax.
Iliac Crest
For posterior procedures, onlay graft material may be needed to supplement the fusion construct. The posterior superior iliac spine is readily palpated in the prone position. To harvest cancellous graft, an incision can be made directly over the posterior superior iliac spine. The fascia over the posterior superior iliac spine is then opened with electrocauterization. An opening in the cortical surface is made with a chisel or gouges. Cancellous bone can be obtained using curettage. Bone bleeding is controlled with Gelfoam and bone wax. The defect can be filled with allograft, or the fascia can be closed with sutures.
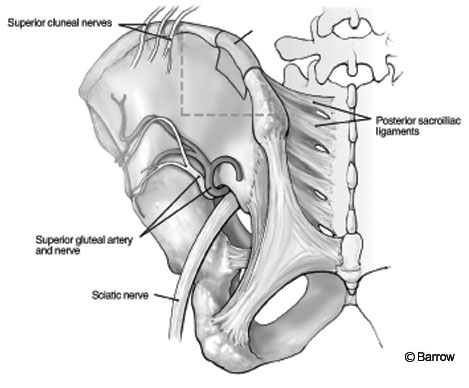
o obtain a corticocancellous graft, a longer exposure is used. The incision for the exposure of the posterior iliac crest should not exceed 8 cm from the posterior superior iliac spine to avoid injury to the superior cluneal nerves, which course over the crest. The fascia over the crest is exposed and opened. The musculature is dissected free using subperiosteal technique. The dissection should not extend too far inferiorly to avoid jeopardizing the structures in the region of the sciatic notch. Externally, the sciatic nerve, superior gluteal nerves, and branches of the superior gluteal artery are present. Internally, the superior gluteal artery and ureter are of concern (Fig. 5).
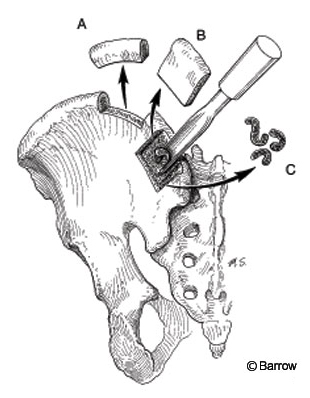
Figure 6. A variety of bone grafts can be harvested from the posterior ilium: (A) tricortical strut graft, (B) cortico-cancellous plate, and (C) cancellous bone strips.
The subcrestal approach is an alternative method for obtaining unicortical and cancellous grafts (Fig. 6). An incision 1 cm lateral to the posterior superior iliac spine allows exposure as described above. Instead of simply perforating the surface of the cortex, however, a unicortical window can be cut with chisels or a saw. Additional cancellous bone can then be harvested through the same opening. Care should be exerted during closure of the fascial layer to avoid damage to the gluteal musculature. With meticulous hemostasis, a postoperative drain should be unnecessary.
Occipital Calvaria
Traditionally, dorsal occipitocervical fusion has required a separate donor site for bone graft. Occipital calvarial bone is an alternative to rib and iliac crest. It can be obtained through the same incision as used for the procedure and is associated with a low rate of complications. With the patient prone, the standard incision and dissection are performed. If occipitocervical fusion is necessary, the skull should be exposed so that an area over the external occipital protuberance is visible. A reciprocating sagittal saw with a 3-mm depth cutter is used in combination with a curved osteotome to cut the outer table of the skull and to lift strips of bone. The diploic cancellous bone below also can be harvested with curettes or gouges. The inner table of the skull must not be perforated to avoid plunging intracranially. This bone can be used as strips placed in the region to be fixated, or it can be morcellized and packed into the fusion mass (not shown). Alternatively, a full thickness graft can be obtained using a craniotome. Parietal bone can be used for a split thickness bone graft (Fig. 7).

Conclusions and Future Developments
The use of harvested autograft in spinal fusion is well established. It represents the most ideal graft material for spinal arthrodesis because it has the three major features needed to encourage fusion: osteogenesis, osteoinduction, and osteoconduction. The development of synthetic BMP represents the beginning of a new age in spinal arthrodesis. Genetically engineered materials may soon replace harvested bone, eliminating the potential for morbidity and maximizing surgical outcomes.
References
- Albee FH: Transplantation of a portion of the tibia into the spine for Pott’s disease. JAMA 57:885-886, 1911
- Asano S, Kaneda K, Satoh S, et al: Reconstruction of an iliac crest defect with a bioactive ceramic prosthesis. Eur Spine J 3:39-44, 1994
- Banwart JC, Asher MA, Hassanein RS: Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine 20:1055-1060, 1995
- Chan DPK, Whitesides TE, Spetzler RF: Surgical approaches to the spine and bone grafts in spinal surgery, in Evarts CM (ed): Surgery of the Musculoskeletal System, 2nd ed. New York: Churchill Livingstone, 1990, pp 1817-1855
- Gore DR: The arthrodesis rate in multilevel anterior cervical fusions using autogeneous fibula. Spine 26:1259-1263, 2001
- Hardy JH: Iliac crest reconstruction following full-thickness graft: A preliminary note. Clin Orthop 123:32-33, 1977
- Harris MB, Davis J, Gertzbein SD: Iliac crest reconstruction after tricortical graft harvesting. J Spinal Disord 7:216-221, 1994
- Kalfas IH: Principles of bone healing. Neurosurg Focus 10:Article 1, 2001
- Kim CW, Abrams R, Lee G, et al: Use of vascularized fibular grafts as a salvage procedure for previously failed spinal arthrodesis. Spine 19:2171-2175, 2001
- Kurz LT, Garfin SR, Booth RE: Iliac bone grafting: Techniques and complications of harvesting, in Garfin SR (ed): Complications of Spine Surgery. Philadelphia: J. B. Lippincott, 1986, pp 323-341
- Wang MY, Levi ADO, Shah S, et al: Polylactic acid mesh reconstruction of the anterior iliac crest after bone harvesting reduces early postoperative pain after anterior cervical fusion surgery. Neurosurgery 51:413-416, 2002
