
The Role of Eye Movements During Visual Fixation*
Susana Martinez-Conde, PhD
Division of Neurobiology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
*Courtesy of Susana Martinez-Conde, PhD
The Martinez-Conde Laboratory: Susana Martinez-Conde received her PhD from the University of Santiago de Compostela, Spain. She completed a postdoctoral position at Harvard Medical School with David Hubel, who received the Nobel Prize for his studies in the visual system. Dr. Martinez-Conde then established her own laboratory at University College London (United Kingdom) before joining Barrow. Xoana Troncoso received her BS in physics from the University of Santiago de Compostela, Spain. She is currently enrolled as a PhD student at University College London. Shannon Bentz (Research Assistant) received his BA from Florida State University. He previously worked at the University of Maryland and at Wake Forest University. Thomas Dyar (Senior Programmer/Data Analyst) received his BA from Boston University and worked at Massachusetts Institute of Technology. Alex Schlegel (Programmer/Data Analyst) received his BS from North Carolina State University. He then worked at Dartmouth College and University College London. Veronica Shi and Elizabeth Do are junior volunteers in the Barrow Student Enrichment Program.
Abstract
Our eyes continually move even while we fixate our gaze on an object. Although fixational eye movements have a magnitude that should make them visible to us, we are unaware of them. If fixational eye movements are counteracted, our visual perception fades completely due to neural adaptation. Thus our visual system has a built-in paradox. We must fixate our gaze to inspect the minute details of our world; yet, if we were to fixate perfectly, the entire world would fade from view. Their role in counteracting adaptation makes fixational eye movements an important tool for investigating how the brain makes the environment visible. By studying fixational eye movements, we hope to understand the visual processing that occurs during fixation, both in normal vision and in patients with oculomotor disease.
Key Words: adaptation, awareness, blindness, eye movements, fading, microsaccades, visibility, vision
We live in a constantly changing world, and most (if not all) nervous systems have evolved to detect changes in the environment. Motion in our visual field may indicate that a predator is approaching or that prey is escaping. Stationary objects usually pose less of a threat. They therefore tend to be studiously ignored due to the brain’s neuronal adaptation mechanisms.
Some nervous systems are specialized to detect only motion signals (i.e., a frog may not see a resting fly but reacts as soon as the fly takes off). In a sense, this ability to see only moving objects may be true of all visual systems. Even our own visual system can detect stationary objects only because the images projected onto our retinas are never stationary for long: Even during visual fixation, our eyes are often moving.
Fixational eye movements have been known for many years. In 1738 Jurin referred to the trembling of the eye. In 1867 Helmholtz admitted the difficulty of maintaining perfect fixation and proposed that this “wandering of the gaze” served to prevent retinal fatigue.[5] In the early 1950s, the development of methods to counteract eye movements and thus cause visual fading led to considerable research intended to characterize the eye movements that occur during fixation. In the late 1970s, however, the field of fixational eye movements seemed to arrive at an impasse. Interest in fixational eye movements began to wane because of difficulties in collecting data, discrepancies in results from different laboratories, and disagreements about the interpretation of the available data.
In the late 1990s interest was revived by the development of accurate techniques for measuring eye movements and by the advent of single-unit recording techniques in alert monkeys. For the first time, it was possible to ask what types of neuronal responses were generated by eye movements during visual fixation. By correlating neural activity with fixational eye movements (which are themselves correlated with the maintenance of visual perception), our laboratory and others have begun to address how visual information is encoded in the brain during visual fixation.[6-8] There are three primary types of eye movements during visual fixation in primates: tremor, drifts, and microsaccades. Of these three types of fixational eye movements, microsaccades are the largest and easiest to characterize. This article focuses on the physiological and perceptual contributions of fixational microsaccades.
Microsaccade Parameters
Fixational microsaccades are small, fast, jerk-like eye movements that occur during voluntary fixation. They carry the retinal image across the widths of several dozen to several hundred photoreceptors and last about 25 msec. Microsaccades have been described in several species other than humans.[1] They seem to be most important, however, in species with foveal vision (such as monkeys and humans).
A possible role for microsaccades is to correct displacements in eye position produced by drifts of the eye during fixation. For example, if drift carries the fixation target away from the fovea, microsaccades tend to bring the target back. Recent studies suggest that microsaccades may counteract receptor adaptation on a short timescale and correct fixation errors on a longer timescale.[14]
Microsaccades and voluntary saccades may be generated by the same mechanisms[13] (i.e., circuits leading to saccade-related burst neurons in the superior colliculus[11]). However, unlike the larger voluntary or exploratory saccades, microsaccades cannot be elicited voluntarily. The involuntary nature of microsaccades may indicate a subcortical control mechanism for their production. Microsaccades in the two eyes tend to be conjugate.[12] Therefore, different neural mechanisms may be involved in the generation of voluntary saccades and involuntary microsaccades.
Microsaccades and Visual Fading
The human visual system is governed by neural adaptation. Steady illumination produces weak neural responses, whereas abrupt changes in illumination across space and time generate strong responses. In this sense, neural adaptation is the cornerstone of all visual processing.
The cost of such a system is that unchanging features of the scene fade from view. Eye movements during fixation are therefore necessary to overcome the loss of vision related to uniform stimulation of the retinal receptors, even at the potential cost of a decrease in visual acuity. The goal of oculomotor fixational mechanisms may not be retinal stabilization but rather controlled image motion adjusted to be optimal for visual processing. In the early 1950s, several independent groups showed that all eye movements could be eliminated in the laboratory, causing visual perception to fade to a homogeneous field.[4,9,12] This finding may seem counterintuitive, but it is a common experience in all sensory modalities: We seldom notice that our shoes are on for 16 hours a day. When the eyes were released from artificial stabilization or if the stabilized image was changed, visual perception reappeared just as we again notice that our shoes are on if we wiggle our toes.
Even though perfect retinal stabilization is most easily achieved under laboratory conditions, objects fade in our visual periphery quite often in normal vision. We are usually unaware of the process. Peripheral fading of stationary objects was first noticed by Troxler in the early 1800s (Fig. 1). He reported that, under voluntary fixation, stationary objects in the periphery of vision tend to fade and disappear.[10] In the late 1950s, Clarke made a connection between Troxler fading and the fading of stabilized images in the laboratory and attributed both phenomena to neural adaptation.[2,3] The simplest explanation for Troxler fading is that receptive fields in the periphery of our vision can be considerably larger than fixational eye movements, especially because accurate fixation tends to eliminate microsaccades. Drifts and tremor, which are smaller in magnitude than peripheral receptive fields, may not provide effective visual stimulation to prevent peripheral visual fading, especially in the case of low-contrast stimuli.
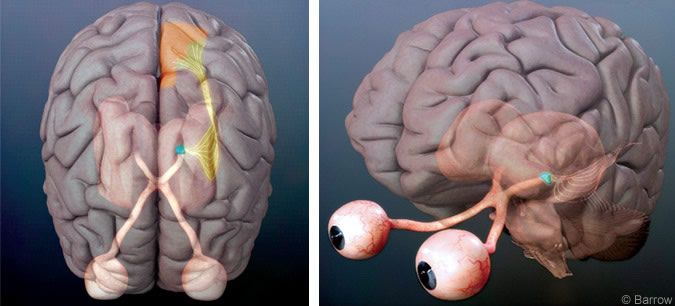
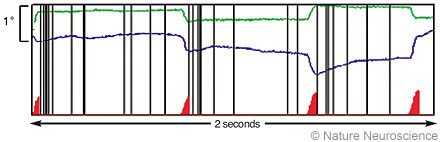
Figure 3. Correlation between microsaccades and bursts of spikes in primate area V1 during a 2-second period. The green and blue traces represent horizontal and vertical eye positions, respectively (tracked with a search coil). We identified microsaccades objectively with an automatic algorithm. The red triangles indicate where a microsaccade has occurred (the height of the triangles represents magnitudes of microsaccades). The vertical black lines represent the spikes of a single V1 neuron. Microsaccades tend to be followed by a rapid cluster, or burst, of spikes. From Martinez-Conde S, Macknik SL, Hubel DH: Microsaccadic eye movements and firing of single cells in the striate cortex of macaque monkeys. Nat Neurosci 3:251-258, 2000. With permission from Nature Neuroscience.
Neural Responses to Microsaccades
The neural responses to fixational eye movements have been studied in the retina,[15] lateral geniculate nucleus,[7] cortical area V1[6,7] and extrastriate cortex.[16] In our experiments, macaque monkeys were usually trained to fixate their gaze on a small fixation spot. A stationary stimulus of optimal characteristics (for instance, a bar with optimal dimensions and orientation for recording from area V1) was placed over the receptive field of the neuron being recorded. Microsaccades were then correlated with subsequent neural activity. Because the visual stimulus did not move and the head was fixed, neural activity was modulated only when fixational eye movements moved the visual receptive field over the stationary stimulus.
Microsaccades were predominantly excitatory in the lateral geniculate nucleus and area V1 (Figs. 2-4).[6,7] Neuronal responses after microsaccades were purely visual: Microsaccades led to an increased neural activity when a stationary bar of light was centered over the neuron’s receptive field. However, when the bar was removed from the receptive field (and the monitor facing the monkey was blank except for the fixation cross), microsaccades caused no changes in neural activity.
This lack of response demonstrated that microsaccade-induced activity in early visual neurons was visual rather than motor. These neurons were excited only when their receptive fields swept across stationary stimuli. They were not excited during equivalent action by the motor system in the absence of a visual stimulus (Fig. 4A).[6,7] Presumably, microsaccades first generate neural signals at the level of retinal photoreceptors by moving the receptive fields of the photoreceptors over otherwise stationary stimuli. This photoreceptor activity would then be transmitted to subsequent levels in the visual hierarchy.
To address the effectiveness of microsaccades in generating neural activity, we compared neural responses induced by microsaccades to neural responses induced by flashing bars. Onset responses to flashing bars in the lateral geniculate nucleus and cortical area V1 were about seven times larger than the responses to stationary bars moved across the receptive fields of the neurons by microsaccades. Perhaps, the difference can be attributed to the relative abruptness of flashes as stimuli (Fig. 4C and D).[7] The optimality of the stationary visual stimulation had an effect on the size of the response after microsaccades. When the stationary stimulus covering the receptive field of the neuron had optimal characteristics (for instance, a bar of light with optimal orientation), microsaccades during fixation generated larger responses. When the stimulus centered on the receptive field had nonoptimal characteristics, microsaccades induced smaller responses (Fig. 4B).[7]
In summary, microsaccades produced during visual fixation prevent neural adaptation. They do so, at least partially, by inducing visual neurons to keep firing to stationary stimuli. Neural responses to microsaccades are an important clue to the “language” our brain uses to represent the visibility of the world.
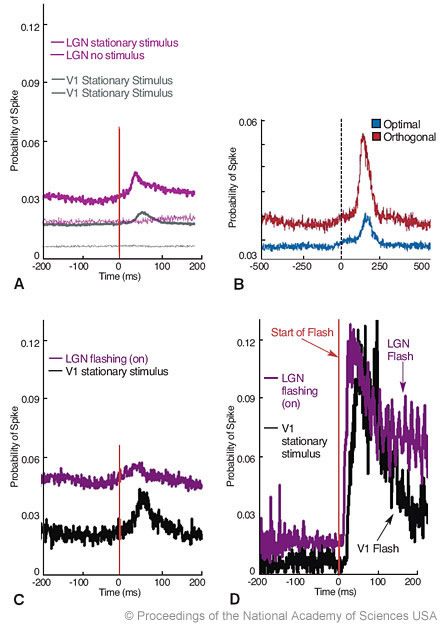
Figure 4. (A) Neural responses to microsaccades. Microsaccades increase spike probabilities in the lateral geniculate nucleus (LGN, n = 57 cells) and V1 (n = 308 cells) in the awake monkey. In the absence of visual stimulation, microsaccades do not generate spikes in the LGN (n = 42 cells) or in V1 (n = 37 cells). (B) Average probability of spikes after microsaccades in V1, for optimal (red) and orthogonal (blue) orientations (n = 11 neurons; each cell was tested in both the optimal and orthogonal conditions). (C) Microsaccades increase spike probabilities in the LGN (purple trace, n = 48 neurons) and area V1 (black trace, n = 6 neurons) when a bar is flashing. Starts of all microsaccades are aligned at Time=zero (vertical line). (D) The probability of a spike after a flashing bar turns on is about seven times higher than the probability of a spike after a microsaccade when that same flashing bar is on. The same data set from (B) (LGN and V1) replotted and realigned to the onset of the flashing bar at Time=zero (vertical line). From Martinez-Conde S, Macknik SL, Hubel DH: The function of bursts of spikes during visual fixation in the awake primate lateral geniculate nucleus and primary visual cortex. Proc Natl Acad Sci USA 99:13920-13925, 2002. With permission from the National Academy of Sciences of the United States of America.
Translational Relevance
By studying the neural responses to microsaccades, we hope to understand the visual processing that occurs during fixation in the awake primate lateral geniculate nucleus and primary visual cortex. Proc Natl Acad Sci USA 99:13920-13925, 2002. With permission from the National Academy of Sciences of the United States of America. fixation, both in normal vision and in patients with oculomotor defects. Impaired fixational eye movements can be observed in patients with central (i.e., brainstem patients) or peripheral pathologies (i.e., dystrophies of extraocular muscles). Evidence from my laboratory and others suggests that fixational eye movements are critical to maintain visual perception during fixation of stationary stimuli. Using perceptual and physiological approaches to understand the contributions of fixational eye movements to normal visual function may be important to helping restore visual function in patients who cannot produce normal fixational eye movements.
References
- Carpenter RHS: Movements of the Eyes. London: Pion Ltd, 1988
- Clarke FJJ: Rapid light adaptation of localised areas of the extra-foveal retina (Troxler’s effect). Optica Acta 4:69-77, 1957
- Clarke FJJ: A study of Troxler’s effect. Optica Acta 7:219-236, 1960
- Ditchburn RW, Ginsborg BL: Vision with a stabilized retinal image. Nature 170:36-37, 1952
- Helmholtz H: Helmholtz’s Treatise on Physiological Optics. (Transl. from 1867 ed.) Birmingham: Gryphon Editions Ltd., 1985
- Martinez-Conde S, Macknik SL, Hubel DH: Microsaccadic eye movements and firing of single cells in the striate cortex of macaque monkeys. Nat Neurosci 3:251-258, 2000
- Martinez-Conde S, Macknik SL, Hubel DH: The function of bursts of spikes during visual fixation in the awake primate lateral geniculate nucleus and primary visual cortex. Proc Natl Acad Sci U S A 99:13920-13925, 2002
- Martinez-Conde S, Macknik SL, Hubel DH: The role of fixational eye movements in visual perception. Nat Rev Neurosci 5:229-240, 2004
- Riggs LA, Ratliff F: The effects of counteracting the normal movements of the eye. J Opt Soc Am 42:872-873, 1952
- Troxler D: Ueber das Verschwinden gegebener Gegenstande innerhalb unseres gesichtskreises, in Himly K, Schmidt JA (eds): Ophthalmologische Bibliothek. Jena: Springer, 1804, pp 1-53
- Wurtz RH: Vision for the control of movement. The Friedenwald Lecture. Invest Ophthalmol Vis Sci 37:2130-2145, 1996
- Yarbus AL: Eye Movements and Vision. New York: Plenum Press, 1967
- Zuber BL, Stark L: Microsaccades and the velocity-amplitude relationship for saccadic eye movements. Science 150:1459-1460, 1965
- Engbert R, Kliegl R: Microsaccades keep the eyes’ balance during fixation. Psychol Sci 15:431-436, 2004
- Greschner M, Bongard M, Rujan P, et al: Retinal ganglion cell synchronization by fixational eye movements improves feature estimation. Nat Neurosci 5: 341-347, 2002
- Bair W, O’Keefe LP: The influence of fixational eye movements on the response of neurons in area MT of the macaque. Vis Neurosci 15: 779-786, 1998
Related Readings
- Martinez-Conde S, Macknik SL, Hubel DH: Microsaccadic eye movements and firing of single cells in the striate cortex of macaque monkeys. Nat Neurosci 3:251-258, 2000
- Martinez-Conde S, Macknik SL, Hubel DH: The function of bursts of spikes during visual fixation in the awake primate lateral geniculate nucleus and primary visual cortex. Proc Natl Acad Sci U S A 99:13920-13925, 2002
- Martinez-Conde S, Macknik SL, Hubel DH: The role of fixational eye movements in visual perception. Nat Rev Neurosci 5:229-240, 2004
- Troncoso XG, Macknik SL, Martinez-Conde S: Novel visual illusions related to Vasarely’s ‘nested squares’ show that corner salience varies with corner angle. Perception 34:409-420, 2005
