
Staged Surgical and Endovascular Treatment of a Giant Serpentine Basilar Artery Aneurysm: Case Report
Authors
Paul J. Apostolides, MD
Michael T. Lawton, MD
Jeff W. Chen, MD, PhD†
John McKenzie, MD‡
Robert F. Spetzler, MD
Division of Neurological Surgery, ‡Barrow Neurological Institute, Mercy Healthcare Arizona, Phoenix, Arizona
Current Address: †University of Texas, Medical Branch at Galveston, Texas, ‡Baptist Hospital, Jacksonville, Florida
Abstract
A giant serpentine artery arising from the trunk of the basilar artery is treated successfully with staged surgical and endovascular therapy. Giant aneurysms sometimes demand a combination of surgical and endovascular techniques for successful management. This case represents the first report of a giant serpentine basilar artery aneurysm treated with staged intracranial-extracranial bypass and permanent basilar artery occlusion using a detachable balloon. The patient enjoyed a good long-term functional outcome.
Key Words : balloon occlusion, basilar artery, cerebral revascularization, endovascular therapy, extracranial-intracranial bypass, giant serpentine aneurysm, subarachnoid hemorrhage
The surgical management of giant serpentine aneurysms of cerebral vessels remains a complex and challenging problem for neurosurgeons. Definitive treatment of these lesions often requires alternative (nonclip) techniques because their location, large size, or lack of a discrete neck prohibits either direct surgical approach or conventional clipping.3-5,21,24 We report the successful treatment of a giant serpentine aneurysm arising from the trunk of the basilar artery using staged surgical and endovascular therapy.
Case Report
A 45-year-old right-handed male with long-standing, poorly controlled hypertension presented with a 1-year history of progressive left-sided weakness, dysarthria, and gait difficulty. The patient”s physical examination was remarkable for bilateral endgaze nystagmus on far lateral gaze, a left pronator drift, and 4/5 strength of the left upper and lower extremities. He demonstrated left-sided dysmetria and dysdiadochokinesia and had a positive Romberg” s sign. He had 3+ reflexes and an upgoing toe on the left and was ambulatory with the assistance of a cane.
Radiologic Studies
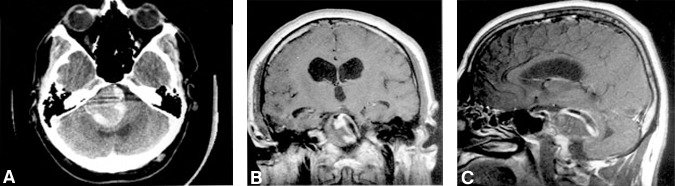
A computed tomography scan of the head demonstrated a 3.5- to 4.0-cm diameter calcified lesion with an isointense central region in the posterior fossa that was causing significant brain stem compression and hydrocephalus (Fig.1A). A peripheral rim of enhancement was seen after the administration of intravenous contrast agent. T1-weightedaxial, coronal, and sagittal magnetic resonance images revealed a 3.5- to 4.0-cm diameter lesion with heterogenous signal characteristics. A flow void was clearly visible within the lesion (Fig. 1B and C).
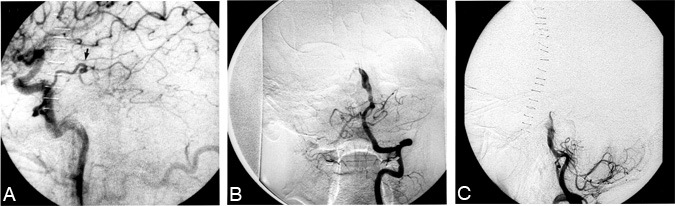
Cerebral angiography (Fig. 2A, B, and C) revealed a giant aneurysm arising from the basilar trunk between the anterior inferior cerebellar arteries (AICAs) and the superior cerebellar arteries. The contrast agent entered the aneurysm just distal to the AICAs, ascended, followed a serpentine course to the patient”s right, and returned to the midline joining the basilar artery just below the level of the superior cerebellar arteries. A distinct aneurysmal neck was not visible, and the flow through the serpentine channel was sluggish. There was nocollateral filling of the posterior circulation via the posterior communicating arteries.
Treatment
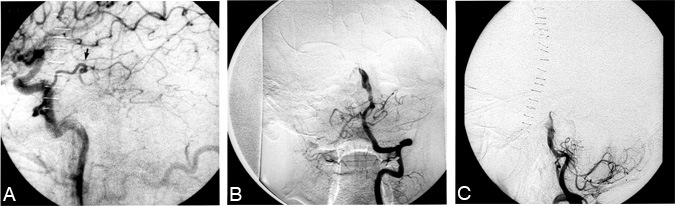
The patient first underwent a left-sided superficial temporal artery-to-superior cerebellar artery bypass (Stage1). A ventriculostomy was placed at surgery and later converted to a ventriculoperitoneal shunt. Postoperative angiography of the left common carotid artery demonstrated a patent bypass with good filling of the superior cerebellar artery (Fig. 3A). A vertebral artery injection showed marked reduction of contrast agent flowing through the aneurysmalsac (Fig. 3B and C).
The patient then underwent a left-sided retrolabyrinthine/presigmoid approach to the basilar artery with the intention of trapping the aneurysm (Stage 2). Additional exposure was obtained by dividing the tentorium. The aneurysm appeared to arise from the basilar artery along its length from the AICAs to the superior cerebellar arteries. A discrete neck was not visualized, and the aneurysm could not be safely trapped because of its proximity to the AICAs and superior cerebellar arteries. During a brief postoperative hospital stay, the patient was allowed to recover and then improved further during a 2-week course of intensive inpatient rehabilitation.
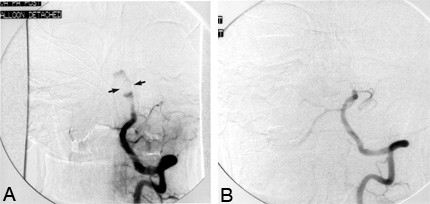
Repeat cerebral angiography revealed the presence of residual flow through the aneurysm. At this time, a detachable balloon catheter was placed in the basilar artery above the level of the AICAs via a transfemoral approach (Stage 3, Fig. 4A). After a 30-minute test occlusion, the balloon was detached and the basilar artery was occluded permanently. The patient tolerated this procedure and again returned to the rehabilitation unit after a brief period of hospitalization. Follow-up cerebral angiography 2 weeks after the balloon occlusion showed no flow through the basilar artery above the level of the AICAs and no residual flow through the aneurysm (Fig. 4B).
The patient then underwent a right retrosigmoid/suboccipital craniotomy and debulking of the thrombus within the aneurysmal sac (Stage 4). The aneurysm was incised between the fifth and seventh/eighth cranial nerve complexes, and considerable soft thrombus was easily removed. Postoperatively, the patient did well. He underwent another short course of inpatient rehabilitation and returned home. At a 36-month follow-up examination, he was fully independent. His strength was normal and he had minimal residual cerebellar signs. He was walking 3 to 5miles a day and was employed.
Discussion
Giant serpentine aneurysms are rare lesions most often found on branch vessels of the middle cerebral artery (MCA).6,11,21 They are characterized by a tortuous vascular channel that travels withinthe body of a thrombosed aneurysm. The channel has distinct entrance and exit sites3,6,11,15,16 andis associated with slow circulation.11,15 The growth of these lesions is thought to result from jet-flowphenomena that can occur when blood enters an aneurysm that is much larger than its orifice.3,11 Asthe blood flows away from the orifice, differential pressure zones are created and promote the development of avascular channel within the thrombus as well as progressive enlargement of the aneurysm. The territory of the MCA appears to be particularly well-suited for the development of serpentine aneurysms because there are few surrounding structures to prevent their growth.3,15
The treatment of giant serpentine aneurysms remains formidable because their large size, surgical in accessibility, or lack of a discrete neck can make conventional clipping infeasible. Alternative treatments often necessitate sacrifice of the parent vessel and therefore risk ischemic complications. Tests to determine tolerance such as the balloon occlusion test can be useful but have associated risks of neurological morbidity that rangefrom 0.7% to 7%.14,22 In addition, the risks of ischemic complications after basilar artery occlusion are considerable.2 In the experience of Drake and coworkers19 with basilar artery occlusion in 46 patients with basilar trunk aneurysms, 10 patients (21.6%) deteriorated neurologically, 6 (13%) from ischemia. Furthermore, the risk of delayed ischemic deficits is not predicted by test occlusion.12 Of Drake” sentire series of 201 patients, 35 (17.4%) experienced ischemic deficits, of which 21 were delayed (1 hour to 10 days). Late ischemic complications observed in another six patients (3%) during an 18-month follow-up period included three transient ischemic attacks and three vertebrobasilar strokes (fatal in two patients).
Many authors have concluded that the presence of significant collateral circulation to the rostral brain stem through the posterior communicating arteries is critical for tolerating basilar artery occlusion andhave suggested that revascularization may be important in the surgical management of these patients.1,9,10,18-20 In our experience, the morbidity resulting from revascularization is low.12 Therefore, we favor revascularization with basilar artery occlusion when the adequacy of collateral blood flow from the posterior communicating arteryis questionable. In addition to augmenting the cerebrovascular reserve to distal basilar artery territories, the bypass can alter the hemodynamics in the aneurysm and promote thrombosis of the lumen. These findings were observedin our case after superficial temporal artery-to-superior cerebellar artery bypass. However, thrombosis was incomplete and endovascular balloon occlusion of the basilar artery was required to eliminate the aneurysm. A preexisting occlusion of the perforating arteries arising from the aneurysm by spontaneous partial thrombosis of the aneurysm may contribute to tolerance of basilar artery occlusion.1
Permanent detachable balloon occlusion of the basilar artery with a good patient outcome hasnot been reported in a patient with a giant serpentine basilar artery aneurysm. In 1974, Serbinenko17reported the first two cases of permanent balloon occlusion of the basilar artery—one to treat a patient with abasilar artery aneurysm and the other to treat a basilar artery-to-basilar plexus fistula. Both patients, however, died. Hieshima and colleagues7 reported the successful treatment of a 23-year-old male with a large midbasilar artery aneurysm by placing two detachable silicone balloons into the dome of the aneurysm; the balloons occluded both the midbasilar artery and the aneurysm. Higashida and coworkers8 likewise successfully treated a 23-year-old female with a large ectatic (and serpentine) midbasilar artery aneurysm with both aneurysm and parent-vessel occlusion using detachable balloons. Their only patient with a giant mid basilar artery aneurysm, a 10-year-old female, also appeared to be treated with detachable balloon occlusion of both the aneurysm and midbasilar artery. However, she developed transient cerebral ischemia and died 2 months after the procedure from rupture of a second untreated aneurysm.
Aymard and colleagues1 reported the first successful balloon occlusion of the proximal basilar artery to treat a 13-year-old male with a giant fusiform aneurysm of the basilar artery. However, the balloon was not detached from the catheter because it migrated distally into the aneurysm during inflation. Hodes and coworkers9 reported treating a patient with a giant saccular aneurysm of the basilar artery with balloon occlusion of the parent vessel. This patient developed hemiplegia and oculomotor paralysis 2 hours after occlusion and subsequently died from a pontine hemorrhage.
Compression of neural structures by the aneurysmal mass can cause many of the complications inpatients with giant intracranial aneurysms, particularly those involving the basilar trunk.19,23 This neural compression can persist long after the aneurysm has been eliminated from the circulation1 and may be responsible for persistent symptoms. Therefore, we often debulk these aneurysms after their complete exclusion from the circulation to relieve mass effect and hasten recovery.13 Clipped or trapped aneurysms are debulked immediately; proximally occluded aneurysms are debulked in a delayed fashion after angiographic verification of complete thrombosis. The goal of aneurysm debulking is reduction of mass effect and not complete removal of the thrombus or aneurysmal dome. Portions of the aneurysm that adhere to the brain stem or delicate surrounding structures are better left alone rather than risk neurologic injury by their complete removal. In addition to the debulking, division of the tentorium can help decompress the posterior fossa and brain stem. Soon after aneurysmal debulking, our patient”s symptoms improved dramatically with increased left-sided strength, decreased cerebellarsigns, and decreased dysarthria.
This report illustrates the significant complexity of treating patients with giant aneurysms of the basilar trunk and highlights the potential effectiveness of staged surgical and endovascular therapy in managing these formidable lesions. Our case and similar cases reported by other authors1,7-9 also suggest that endovascular balloon occlusion of the basilar artery may represent a practical technique of proximal occlusion in carefully selected patients.
References
- Aymard A, Hodes JE, Rüfenacht D, et al: Endovascular treatment of a giant fusiform aneurysmof the entire basilar artery. AJNR 13:1143-1146, 1992
- Drake CG: Ligation of the vertebral (unilateral or bilateral) or basilar artery in the treatment of large intracranial aneurysms. J Neurosurg 43:255-274, 1975
- Fodstad J, Liliequist B, Wirell S, et al: Giant serpentine intracranial aneurysm after carotid ligation. Case report. J Neurosurg 49:903-909, 1978
- Fukamachi A, Hirato M, Wakao T, et al: Giant serpentine aneurysm of the posterior cerebralartery. Neurosurgery 11:271-276, 1982
- Greene KA, Anson JA, Spetzler RF: Giant serpentine middle cerebral artery aneurysm treated by extracranial-intracranial bypass. Case report. J Neurosurg 78:974-978, 1993
- Haddad GF, Haddad FS: Cerebral giant serpentine aneurysm: Case report and review of the literature.Neurosurgery 23:92-97, 1988
- Hieshima GB, Higashida RT, Wapenski J, et al: Intravascular balloon embolization of a large mid-basilar artery aneurysm. Case report. J Neurosurg 66:124-127, 1987
- Higashida RT, Halbach VV, Cahan LD, et al: Detachable balloon embolization therapy of posterior circulation intracranial aneurysms. J Neurosurg 71:512-519, 1989
- Hodes JE, Aymard A, Gobin YP, et al: Endovascular occlusion of intracranial vessels for curative treatment of unclippable aneurysms: Report of 16 cases. J Neurosurg 75:694-701, 1991
- Hopkins LN, Budny JL, Castellani D: Extracranial-intracranial arterial bypass and basilar artery ligation in the treatment of giant basilar artery aneurysms. Neurosurgery 13:189-194, 1983
- Kumabe T, Kaneko U, Ishibashi T, et al: Two cases of giant serpentine aneurysm. Neurosurgery 26:1027-1033, 1990
- Lawton MT, Hamilton MG, Morcos JJ, et al: Revascularization and aneurysm surgery: Current techniques, indications, and outcome. Neurosurgery 38:83-94, 1996
- Lawton MT, Spetzler RF: Surgical management of giant intracranial aneurysms: Experience with171 patients. Clin Neurosurg 42:245-266, 1995
- Origitano TC, Al-Mefty O, Leonetti JP, et al: Vascular considerations and complications in cranial base surgery. Neurosurgery 35:351-363, 1994
- Patel DV, Sherman IC, Hemmati M, et al: Giant serpentine intracranial aneurysm. Surg Neurol 16:402-407, 1981
- Segal HD, McLaurin RL: Giant serpentine aneurysm. Report of two cases. J Neurosurg 46:115-120,1977
- Serbinenko FA: Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg 41:125-145, 1974
- Spetzler RF: Comments on Hopkins et al: Extracranial-intracranial arterial bypass and basilar artery ligation in the treatment of giant basilar artery aneurysms. Neurosurgery 13:194, 1983
- Steinberg GK, Drake CG, Peerless SJ: Deliberate basilar or vertebral artery occlusion in the treatment of intracranial aneurysms. Immediate results and long-term outcome in 201 patients. Neurosurgery 79:161-173, 1993
- Sundt TM Jr., Piepgras DG, Houser OW, et al: Interposition saphenous vein grafts for advanced occlusive disease and large aneurysms in the posterior circulation. J Neurosurg 56:205-215, 1982
- Suzuki S, Takahashi T, Ohkuma H, et al: Management of giant serpentine aneurysms of the middle cerebral artery. A review of literature and report of a case successfully treated by STA-MCA anastomosis only.Acta Neurochir (Wien) 117:23-29, 1992
- Tarr RW, Jungreis CA, Horton JA, et al: Complications of preoperative balloon test occlusion of the internal carotid arteries: Experience in 300 cases. Skull Base Surgery 1:240-244, 1991
- Tomasello F, Albanese V, Cioffi FA: Giant serpentine aneurysms: A separate entity. SurgNeurol 12:429-432, 1979
- Whittle IR, Dorsch NW, Besser M: Giant intracranial aneurysms: Diagnosis, management, and outcome. Surg Neurol 21:218-230, 1984
