
The Therapy of Primary Brain Tumors
William R. Shapiro, MD
Division of Neurology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
Therapy for malignant gliomas is multimodal and includes surgery, radiation therapy, and chemotherapy. Prognostic variables often influence outcome more than therapy; a young age, low tumor grade, and a high Karnofsky performance score are associated with longer survivals. Surgical resection that leaves little residual tumor is associated with longer survivals than less complete resections. Radiation therapy of at least 60 Gy is required to treat malignant gliomas. Increased survival beyond that produced by standard external radiation therapy requires much larger doses, which interstitial radiation therapy permits. The efficacy of radiosurgery is being tested. Chemotherapy with nitrosoureas is modestly useful but appears to benefit patients with anaplastic astrocytomas more so than those with glioblastomas. Therapy for low-grade astrocytomas and oligodendrogliomas includes close observation, surgical resection, radiation therapy, and chemotherapy, depending on when in the “natural history” of the disease the patient presents to the doctor.
Key Words : astrocytomas, chemotherapy, glioblastomas, gliomas, oligodendrogliomas, radiation therapy, tumors
Each year in the United States, approximately 15,000 new cases of adult central nervous system (CNS) tumors are diagnosed, and each year 11,000 deaths are attributed to these tumors.44 Pediatric brain tumors are less numerous, but with approximately 1,200 new cases diagnosed each year, they are now the leading cause of death from cancer in children.65In adults, lower grade gliomas tend to occur in patients between 20 and 40 years old, middle grade gliomas in patients between 40 and 55 years old, and highly malignant gliomas in patients 55 years and older. Some evidence suggests that the incidence of malignant gliomas in patients older than 70 years is increasing,23, 83 but the issue is disputed.58 In children, brain tumors occur most commonly between the ages of 3 and 12 years. Brain tumors affect the sexes differently. Glioma, the most common CNS tumor in adults, is more common in males than in females; the reverse is true for meningiomas. Glioblastomas multiforme (GBMs) occur most frequently, followed equally by meningiomas and astrocytomas. The most common malignant childhood tumors are cerebellar medulloblastomas. The following discussion is limited to gliomas in adults.
A three-tiered classification system places well-differentiated astrocytomas at the low end of the spectrum of malignancy, GBMs at the high end, and anaplastic astrocytomas (AAs) in the middle.9 The distinction between astrocytomas and AAs is based on the higher mitotic rate in the latter. The additional presence of necrosis defines the tumor as a GBM. A newer classification system based on histology appears to correlate well with outcome (Daumas-Duport).14 This system is based on the relative contributions of nuclear abnormalities, mitoses, endothelial proliferation, and necrosis. The World Health Organization (WHO) has used a modification of this system to define tumors in relationship to their aggressiveness.29 Grade I gliomas are usually pilocytic astrocytomas in young people; grade II gliomas include astrocytomas and oligodendrogliomas; grade III gliomas consist of AAs and anaplastic oligodendrogliomas; and grade IV gliomas are glioblastomas.
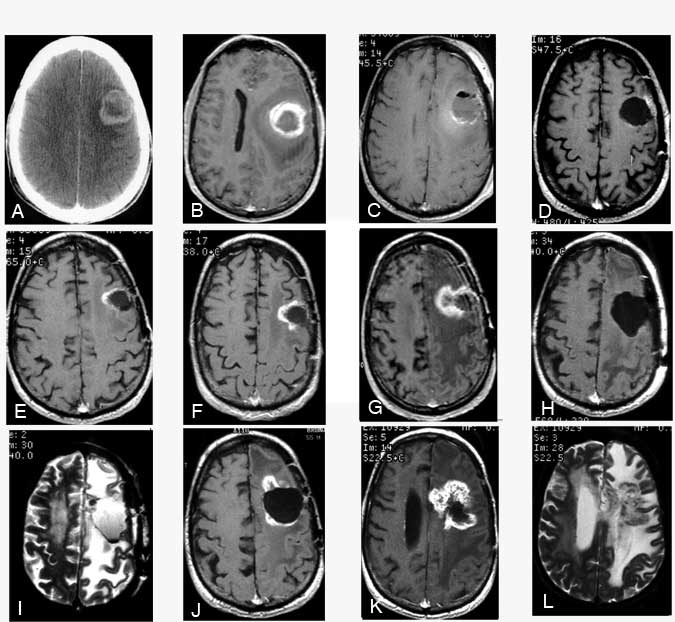
By December 1996, his condition began to decline and he developed mild aphasia. T1-weighted MR imaging with gadolinium showed early enhancement medially (not shown). (E) A T2-weighted image showed edema adjacent to the lesion at the margin of the tumor bed. By March 1997, the enhancing area had enlarged on (F) T1-weighted MR imaging with gadolinium. MR spectroscopy suggested this region was radiation necrosis. (G) T1-weighted MR imaging showed that the region of enhancement had increased substantially. The patient’s aphasia had worsened, and he had developed a severe right hemiparesis. MR spectroscopy again suggested that the enhancing mass was radiation necrosis. The mass was resected on July 15, 1997. Postoperatively, a (H) T1-weighted MR image with gadolinium and (I) a T2-weighted MR image showed the resection bed. Pathological analysis confirmed the diagnosis of radiation necrosis. The patient’s weakness improved as did his speech, although he still had difficulties with word naming. By December 1997, he developed a more profound expressive aphasia, episodic confusion, and moderate right hemiparesis. (J) A T1-weighted MR image with gadolinium revealed further enhancement adjacent to the previous lesion. By February 1998, the patient was confined to his home, taking anticonvulsant medications and modest doses of dexamethasone. (K) A T1-weighted MR image with gadolinium and a (L) T2-weighted MR image showed that the enhancing lesion had increased further as had the area of edema (L), consistent with increasing radiation necrosis.
Malignant Gliomas
Multimodality therapy for malignant gliomas—cytoreductive surgery, radiation therapy, chemotherapy—has been used in one form or another for the past 30 years.62 Much of this experience was a direct product of clinical research by individual institutions and cooperative group trials. Each modality is reviewed briefly, concentrating on recent results and controversies.
Surgical Treatment
Despite 75 years of experience with primary malignant gliomas,47 the role of surgical resection in the treatment of malignant gliomas remains controversial. Surgery permits a pathological diagnosis to be established during life. Many physicians, however, consider that current methods of radiological diagnosis, especially magnetic resonance (MR) imaging, permit a malignant brain tumor to be diagnosed without subjecting the patient to the risks of surgical resection. Stereotactic biopsy provides enough tissue to confirm the diagnosis of primary glioma. However, the amount of tissue obtained from needle biopsies may be inadequate to grade tumors.20Furthermore, stereotactic biopsy alone, or even a small open craniotomy biopsy, denies a role for surgery as “cancer” therapy. With the advent of lasers, surgery has improved enormously. More recently, computer-assisted navigation aids such as the ISG Wand (ISG Technologies, Mississauga, Ontario) have made precise resections possible in patients with malignant glioma.
The surgical reduction of tumor to a very small residual may be associated with prolonged survival. In one Brain Tumor Cooperative Group (BTCG) study, computed tomography (CT) scans from brain tumor patients were studied at several times over the course of their treatment and compared to ultimate outcome.85 Preoperative tumor size and prognosis were not significantly related. In contrast, there was a very strong inverse relationship between postoperative tumor size and length of survival. This trend was especially notable in patients with minimal or no residual enhancing tumor. This beneficial effect of surgery was less the result of debulking than of leaving the least residual tumor possible. Thus, there was no significant relationship between percentage of tumor removed and survival, although patients whose tumors were reduced by 75% or more tended to survive longer.
Albert et al.1 obtained a similar result with postoperative MR imaging (Days 1-3). A significant ordering of survival was associated with removal of more tumor. Patients with postoperative residual contrast-enhanced tumor had almost a seven-fold higher risk of death compared to patients without residual tumor. Postoperative MR imaging was three times more accurate in defining the extent of surgical resection than the surgeon’s estimate. Of the tumor “recurrences,” 80% emerged from contrast-enhanced remnants.
The relationship between extent of surgical resection and survival for glioblastoma was reviewed in three consecutive Radiation Therapy Oncology Group (RTOG) trials.71 Surgical resection was defined as total in 19%, as partial in 64%, and as biopsy only in 17% of the patients. Large surgical resections were associated with statistically significant longer survivals. The median survival of patients with total resection was 11.3 months while that of patients undergoing biopsy was only 6.6 months. Kelly and Hunt27 argue that there is little benefit to attempting major tumor resection in elderly patients, although the survival advantage in their study was always with patients who underwent resection compared to those whose tumors were merely biopsied.
All such retrospective studies are subject to the criticism that the extent of attempted resection depends on the patient’s condition at the time of surgery (e.g., age, tumor location, clinical state) and that favorable conditions usually lead surgeons to attempt larger resections. Therefore, it is unclear whether the extent of surgery or the more favorable prognostic variables are important to survival.
Besides prolonging survival, surgical resection of the tumor (or at least “debulking”) can return many patients to full, active lives without the need for large doses of corticosteroid hormones to ameliorate incapacitating symptoms (Fig. 1A-1C). In a study performed many years ago, tumor-related symptoms improved after tumor resection while new, surgery-related symptoms were uncommon; this result was statistically significant.61 Surgical resection of the tumor often also permits patients to undergo radiation therapy and initial chemotherapy, treatments less likely to be tolerated in the presence of bulky disease. These results support the surgical removal of the largest possible volume of tumor that safe operation allows. There is little justification in performing only a biopsy or limited resection of accessible tumors. If the surgical resection is confined to the tumor itself, major new neurological deficits are rarely produced.
Radiation Therapy
With the advent of better imaging techniques, the appropriate portals and doses of radiation therapy in the treatment of brain tumors have changed. The Brain Tumor Study Group (BTSG)80,81 first reported controlled studies showing that whole-brain radiation therapy increases the survival of patients compared to those undergoing surgery only. Other data showed that patients who received 5500 to 6000 cGy lived significantly longer than those who received 5000 cGy or less.82 In the BTCG study with CT cited earlier,85 patients with no tumor enhancement after radiation therapy survived longer than those with residual tumor. Furthermore, patients with large tumors whose volumes were reduced by more than 50% during radiation therapy survived longer than those whose tumors shrank less than 50% or those whose tumors actually increased.
Radiation portal size was studied in a BTCG trial that compared entirely whole-brain radiation therapy for malignant glioma with whole-brain plus partial coned-down radiation.63 There was no statistical difference in survival among any of the groups, indicating that reduction of the portal size to “boost” the radiotherapy to the tumor volume is as effective as full whole-brain irradiation. Current techniques usually limit the portal size to the volume of the contrast-enhanced tumor plus a 3-cm margin for the entire course of radiotherapy (Fig. 1D). Finally, neither increased fractionation of radiotherapy (twice daily) nor addition of the radiosensitizer misonidazole confers any survival advantage compared to conventional postoperative whole brain radiotherapy and carmustine (BCNU).17 Some researchers believe that higher doses of radiation delivered over a shorter interval, so-called “hypofractionation,” allow patients to receive an adequate radiation dosage in a shorter time.72 In general, we have reserved this form of radiation for elderly patients with large malignant gliomas that cannot be safely resected.
Focal radiotherapy techniques include interstitial implantation of radioactive seeds (brachytherapy) and radiosurgery. Prolonged survival has been reported in patients with recurrent malignant gliomas treated with temporarily implanted sources of 125I.24 BTCG phase III trial 87-01 randomized newly diagnosed patients to receive either (1) postoperative temporary 125I seed implantation in the residual tumor bed followed by standard external beam radiotherapy plus intravenous BCNU or (2) external radiotherapy plus BCNU without the seed implantation. The purpose of this controlled trial was to determine the potential survival value of adding 60 Gy more in the form of brachytherapy to the 60 Gy delivered by external irradiation. Accrual to the study reached 299 patients by April 1994, and the study was closed.
Preliminary review of the results demonstrated that patients who received the 125I seeds tended to live longer than those who did not receive the seeds, although the difference was not quite significant. About 50% of the patients in both the implanted and the nonimplanted groups underwent reoperation. Patients with “recurrent” tumor lived longer after resection if the “recurrence” was due to radiation necrosis (mostly from the seeds) than if tumor was already present at the time of recurrence (Fig. 1E-1L). The incidence of biopsy versus tumor resection was approximately equal in the two groups, thus indicating that the difference in survival was not related to the extent of tumor resection at the time of failure. A “final” evaluation of this study has been completed but not yet published. Although the results favored the interstitial group, they were not statistically significant (p = 0.08). Therefore, the study suggests but does not prove that brachytherapy extends survival beyond that achievable with external radiotherapy alone.
The other technique for delivering local radiation therapy is radiosurgery. Radiosurgery, either by gamma knife or by linear accelerator, is effective in the treatment of arteriovenous malformations, small primary and metastatic brain tumors, and benign brain tumors such as meningiomas and acoustic neuromas. Its investigational use in the treatment of gliomas has been addressed in several reports. One trial used adjuvant radiosurgery as part of the initial management of malignant gliomas.39 Thirty-seven patients received radiosurgery to residual contrast-enhancing tumor (1000 to 2000 cGy) after treatment with conventional external beam radiation therapy. Local recurrence still occurred, but overall survival time may have been longer. Seven (19%) patients required reoperation to remove necrotic tumor at a median length of 5 months after radiosurgery.
A major problem in radiosurgery (as was true for brachytherapy) is selection bias in choosing patients for treatment. Curran et al.13pointed out that radiosurgery-eligible patients live longer than radiosurgery-ineligible patients, when neither group actually receives radiosurgery. Another study revealed little additional survival benefit for malignant gliomas treated with radiosurgery compared to external beam radiotherapy.46 Radiosurgery may benefit a small group of patients with small tumors and good prognoses.40 The RTOG is performing a randomized trial similar to that of the BTCG interstitial radiotherapy study. RTOG 9305 randomizes patients with supratentorial malignant gliomas with a Karnofsky performance status (KPS) ³ 60 and postoperative residual disease of £ 4 cm in greatest diameter to a group that receives radiosurgery followed by external radiotherapy (60 Gy) and BCNU or to a group that receives radiotherapy and BCNU alone.
Chemotherapy
Chemotherapy completes the technique of multimodality treatment of malignant gliomas. In 1983 the BTCG reported that surgery plus radiation therapy and chemotherapy with BCNU significantly increased the survival of patients with malignant gliomas compared to surgery plus radiation therapy without chemotherapy.22 High-dose methylprednisolone does not increase survival.22 Procarbazine and streptozotocin each have been as effective as BCNU.17,22 BCNU alone yields the same results as BCNU sequencing with procarbazine or as BCNU plus hydroxyurea sequencing with procarbazine plus VM-26.63 Intraarterial BCNU is no more effective than intravenous BCNU, but it is substantially more toxic.64 Serious toxicity from intraarterial BCNU includes irreversible encephalopathy, visual loss ipsilateral to the infused carotid artery, or both. In the same study, 5-fluorouracil did not influence survival. Neuropathologically, intraarterial BCNU produced white matter necrosis.59 Intraarterial cisplatin is safer than intraarterial BCNU, but it is no more effective than another nitrosourea PCNU.25
Over the past several years, interest in the use of targeted interstitial drug delivery using biodegradable microspheres and wafers has increased. A controlled trial of such wafers in patients with recurrent malignant gliomas was conducted in a multicenter study.6 Two hundred twenty-two patients requiring reoperation were randomly assigned to receive surgically implanted bio-degradable polymer discs containing 3.85% BCNU or to receive discs without the drug. The median survival of the 110 patients who received BCNU polymers was 31 weeks, significantly longer than the 23-week median survival of the 112 patients who underwent reoperation but received placebo polymers. Because BCNU is readily administered intravenously, a comparative study between BCNU-containing wafers and intravenous BCNU would help define the role of wafers containing this drug. However, studies of wafers containing other agents that do not readily enter brain tumors would be of greater interest.
In addition to these controlled survival-based clinical trials, a large number of agents have also been tested in response-based studies in glioma patients.43 To date, however, no drug has been found to be more effective than the nitrosoureas. A combination of procarbazine (matulane), lomustine (CCNU) and vincristine (PCV) has become a popular chemotherapeutic regimen for malignant glioma and may be more effective than BCNU alone. Of the malignant gliomas, glioblastoma multiforme responds least well to chemotherapy, anaplastic astrocytoma better, and, according to recent studies, oligodendrogliomas may be the most sensitive.10,28
A number of new approaches have been taken toward chemotherapy or, more generally, toward “medical anticancer therapy.” These approaches have included endocrine-related drugs, for example, tamoxifen, a drug used in a low dosage in patients with breast cancer, in which the target is the estrogen receptor. In brain tumors, tamoxifen is used in a very high dosage, and the target is protein kinase C, a large, membrane-based receptor molecule that is active in cellular growth.12,79 Another target is related to angiogenesis, the process whereby the tumor signals to the host to grow new capillaries to support its growth. TNP-470, a compound that blocks such vascular growth,75 is being tested in brain tumor patients. Still another approach is to target intracellular signaling that follows activation of growth receptors. Thus the new drug, SU101, blocks receptor-activated intracellular signals associated with stimulation by platelet-derived growth factor. It too is being tested in brain tumor patients.45 Classical chemotherapy trials are underway with taxol,21,55, 9-aminocamptothecan, etoposide and carboplatin,3 etoposide and intraarterial cisplatin, and the bradykinin analog for opening the blood-brain barrier, RMP-7.5
Future therapeutic strategies include gene therapy-related approaches based on suicide pro-drug administration. One example uses Herpes simplex virus associated with the gene for thymidine kinase (HSV-tK).56 Here, HSV-tK fibroblast vectors from 3T3 fibroblasts are inserted directly into the tumor bed based on the notion that the tumor cells can be induced to produce thymidine kinase through in vivo transection of the genetic information. These cells then are treated with gancyclovir, which essentially forces the cells to commit suicide. The technique is local. The 3T3 fibroblasts must be inserted into the bed of the tumor neurosurgically. There they continue to produce DNA strands, which it is hoped, will ultimately find their way into the tumor cell to make thymidine kinase. In fact, it is not at all clear how much transection occurs, and it has been necessary to hypothesize a “bystander” effect to account for cell death at a distance from the injection site.18 Other gene therapy techniques on the drawing board or ready for clinical trials are also based on this notion of local injection. These techniques include cytidine deaminase systems and antisense treatments.
Low-Grade Gliomas
The advent of MR imaging has substantially improved our ability to diagnose and follow brain tumors. Patients with low-grade gliomas may become symptomatic only with a seizure, but the tumor is readily seen as a mass on T2-weighted MR images. Earlier diagnosis has meant that patients survive for longer periods than when the diagnosis could only be made after invasive diagnostic procedures.54This trend has raised questions about the role of specific therapies. If earlier diagnosis “guarantees” longer survival, how is the efficacy of specific therapy to be judged compared to outcomes before MR imaging was available? Clearly, randomized prospective trials would best judge new treatments; unfortunately, such trials are rare. Some of the published results, however, are reviewed below.
Astrocytomas
Typically, low-grade astrocytomas of the cerebral hemisphere in adults have a good prognosis. Expected survival now approaches 7 to 10 years. Patients with low-grade astrocytomas often become symptomatic with a single seizure, and the tumor is found on MR imaging. However, neither CT nor MR imaging is accurate enough to diagnose or grade such tumors without biopsy. Of 20 patients diagnosed by imaging studies who underwent stereotactic biopsy in one study, only 10 (50%) had low-grade astrocytomas. Nine (45%) had anaplastic astrocytomas and one (5%) had encephalitis.31 Thus, surgical biopsy is necessary to diagnose the tumor and especially to determine its grade.
Therapy for low-grade astrocytomas includes resection, irradiation, and chemotherapy, but when to treat the tumor with which of these modalities has been controversial. Removal may be curative. When, however, residual tumor is present after attempted resection, the patient may (or may not) benefit from radiation therapy. As noted above, a major change in the outcome of treatment of low-grade gliomas has been associated with its earlier diagnosis as made possible by MR imaging. It is difficult to compare the results of therapy in studies that preceded MR imaging with those from studies performed after MR imaging became widely available. Furthermore, recent studies have emphasized multiple prognostic factors that include both pretreatment and treatment variables. Finally, it is not possible to isolate the influence of individual forms of treatment—surgery, radiation therapy, chemotherapy—on outcome, because the different modalities may be used individually or in combination. Several studies are reviewed below. Those with larger numbers of patients are emphasized, beginning with those that focused on surgery.
Laws et al.36 analyzed 461 cases of supratentorial low-grade astrocytomas. Age was the most important prognostic indicator: 83% of patients under 20 years of age, but only 12% of patients 50 years old and over, survived 5 years. Other important prognostic variables included postoperative neurological deficit, altered level of consciousness, type of surgery (actually extent of resection), date of treatment (worse before 1949), and tumor site (frontal/temporal worse). The authors considered resection as the best hope for cure, or at least, to be associated with the longest survival. Radiation therapy appeared to be valuable, primarily in patients over age 40 with more extensive tumors.
Using the newer Daumas-Duport classification, Shaw et al.66 updated the Mayo Clinic experience of Laws, dividing the cases into patients treated between 1960 and 1974 and those treated from 1975 to 1982. The new classification allowed patients originally considered to be Kernohan grades I and II and who had the same prognosis to be divided into four grades with different prognoses. Four variables were identified by multivariate analysis. Of these, the most important was the classification into pilocytic versus ordinaryastrocytoma. The former had 5- and 10-year survival rates of 85% and 79%, respectively, while the latter had 5- and 10-year survival rates of 51% and 23%, respectively. Ordinary astrocytomas included astrocytomas and mixed oligoastrocytomas. Patients with ordinary astrocytomas who received at least 53 Gy of radiotherapy had 5- and 10-year survival rates of 68% and 39%, respectively; corresponding rates for patients who received less than 53 Gy were 47% and 21%. For patients who underwent resection without radiation therapy, the survival rates were 32% and 11%. The retrospective nature of the study precludes definitive conclusions, but the data support the notion that patients with incompletely resected ordinary astrocytomas should receive radiation therapy.
Berger et al.4 reviewed 221 patients at the University of Washington and also found that the extent of surgical resection affects outcome. When the tumors were totally resected, there was no recurrence (mean follow-up, 54 months). Postoperative residual tumor of <10 cm3 was associated with a 14.8% recurrence at 50 months, while larger residual tumor was associated with a 46% recurrence at 30 months (p = 0.002). Furthermore, 46% of patients with residual tumors >10 cm3 had histologically higher-grade recurrences. Radiation therapy, the patients’ ages, and the tumors’ histological subtypes were not prognostic with respect to recurrence. Survival was not analyzed.
In 85 well-differentiated astrocytomas, Soffietti et al.74 reported that total removal was associated with a 5-year survival rate of 51.3% while that associated with subtotal resection yielded only 23.5%. No patient with partial resection survived longer than 5 years. In their review of 55 patients, Piepmeier et al.53 noted that gross total resection in patients who had symptoms longer than 2 years was associated with longer survivals. Indeed, patients with chronic epilepsy lived longer, irrespective of therapy. In contrast, the 10-year survival rate was 100% of 31 patients who underwent gross-total tumor resection, regardless of the duration of preoperative symptoms. In a study from Rome, Scerrati et al.60 reviewed the results of treating 131 patients. As would be expected from a study limited to patients treated mostly in the 1980s, the overall 5- and 10-year survival rates were 97% and 73%, respectively. According to a multivariate analysis, only the extent of surgery was significantly associated with longer survivals.
In contrast to this emphasis on the importance of surgery, Vertosick et al.78 reviewed their experience with 25 patients and concluded that patients live longer than they used to, in part because of the earlier diagnosis made possible by modern imaging. Consequently, they questioned the need for surgical resection. Recht et al.57 found that the overall survival rate of 26 patients who were not operated on for a newly diagnosed, supratentorial, nonenhancing mass found on CT or MR imaging was similar to that of another group of 20 patients who underwent immediate treatment. Overall, the median length of survival for both groups was 84 months.
The role of radiation therapy is also controversial. In most of the studies cited above, patients who could not undergo complete or almost complete resection were usually treated with radiation therapy. Overall success was variable. Some authors reported an advantage for patients treated with radiotherapy (e.g., older or younger). Most authors did not believe that radiation therapy was really necessary if “complete” excision was possible. A variable response could be expected from patients with incomplete resections who were then treated with irradiation.
In contrast, several reports have emphasized radiation therapy as the principal mode of therapy for low-grade astrocytomas. Lunsford et al.42 treated 35 patients with stereotactic biopsy and radiation therapy. Their median survival was 9.8 years, and the authors considered this treatment as an appropriate initial strategy. North et al.50 also noted that radiation therapy was effective. Of 77 operated patients, 66 underwent postoperative radiation therapy. The survival rate of patients treated with 45 to 59 Gy was 66% at 5 years. In a study reported from Japan, Shibamoto et al.70 treated 101 patients with postoperative radiotherapy; 18 patients underwent surgery only. Patients receiving radiotherapy had a 5-year survival rate of 61%, better than that of the patients who underwent surgery only. From Germany, Kreth et al.33 have been using 125I implants as the primary treatment for low-grade gliomas since 1979. Their results demonstrate an overall 5-year survival rate of 60%. They emphasize the influence of several pretreatment prognostic factors (see below). It should be noted that radiation necrosis occurs in a proportion of cases treated with brachytherapy.32 The timing of the radiation therapy may not be an important factor in how patients respond.37 Finally, in one of the few randomized trials of radiation therapy in low-grade gliomas, Karim et al.26 reported the results of a study by the European Organization for Research and Treatment of Cancer that compared 45 Gy with 59.4 Gy. Among 343 eligible and evaluable patients followed for at least 50 months, the difference in survival between those receiving the lower (58%) and higher (59%) doses was not significant.
An early comparison of radiotherapy and delayed radiotherapy in a small cohort of 25 patients found no difference in survival.30 The risk of a Type II error (insufficient number of patients to support equivalency), however, renders the finding only suggestive. A European study examining this question is underway. In Paris, one study has failed to document a decisive role for radiation therapy:5280% of patients with total tumor removal survived 5 years compared with 50% of those undergoing incomplete removal and 45% of those undergoing a biopsy. At 5 years, 65% had survived without radiotherapy compared to 55% of those who underwent radiotherapy.
Winger et al.84 addressed the issue of malignant transformation of low-grade gliomas and its relationship to overall survival. In their study of 285 patients with malignant gliomas, patients with a prior history of low-grade glioma lived significantly longer after the diagnosis of anaplastic glioma than when an anaplastic glioma arose de novo. Thus, early radiation of low-grade gliomas might extend the overall length of survival. One concern about radiating the brains of patients with low-grade gliomas is the potential for radiation damage in a population expected to live long enough to experience the consequences. One report suggests that such damage is uncommon: Specific cognitive deficits were not encountered in patients so treated.76
As alluded to above, several prognostic factors that can substantially affect the survival rates of patients with low-grade astrocytomas can be identified. These factors include a young age,37,48,53,60,70,77 a small tumor,4,33,48,70 a good Karnofsky Performance Scale,33,48 and longer pretreatment symptomotology53 although all studies have not necessarily identified the same prognostic variables. The type of pathology has emerged as an important prognostic variable in that the presence of an oligodendroglioma component may confer a survival advantage (see below).67
Oligodendrogliomas and Oligoastrocytomas
Oligodendrogliomas consist of tumors derived from oligodendrocytes, cells responsible for myelination in the CNS. Oligoastrocytomas are mixed tumors of oligodendroglial and astrocytic cells. Recently, it has been recognized that some astrocytomas are really oligodendrogliomas with reactive astrocytes. However, many astrocytomas, including glioblastomas multiforme, contain oligodendroglial elements. Consequently, it is difficult to evaluate reports of patients whose tumors are called oligodendrogliomas or oligoastrocytomas, especially reports from more than a few years ago. This section emphasizes oligodendrogliomas, and where reported, oligoastrocytomas.
Oligodendrogliomas occur mostly in middle-aged adults, although there is also a small peak of incidence in children.51 Several reports have related survival to grading of oligodendrogliomas. Histologically, grading has been related to number of mitoses and degree of necrosis8 and to endothelial proliferation, necrosis, maximal nuclear/cytoplasmic ratios, maximal cell density and pleomorphism.41,73
In an extensive analysis of their available material from Paris, Daumas-Duport and her colleagues15,16 redefined “pure” oligodendrogliomas into two histological patterns and two clinical patterns. The histological patterns consisted of isolated tumor cells (ITC) and solid tumor tissue. The former infiltrated but did not destroy normal brain parenchyma, while the latter destroyed the brain and formed microblood vessels. Of 153 specimens, two-thirds were exclusively composed of ITCs, a pattern they called structure type III. The other third of the specimens exhibited both ITCs and solid tumor tissue, a pattern called structure type II.
The difference in patterns correlated to contrast enhancement on MR imaging (64% of structure type II, never in structure type III) and to neurological deficit in 57% of the structure type II and in 8% of the structure type III. Seventy-nine cases correlated with MR imaging and CT.15 Contrast enhancement was associated with more aggressive behavior and shorter survivals. When enhancement was present, the median survival was 3 years; without enhancement, it was 11 years. Similarly, endothelial hyperplasia correlated with survival: 3.5 years with hyperplasia and 11 years without hyperplasia.
These observations led the authors to propose a new classification for oligodendrogliomas. Grade A indicates an absence of endothelial hyperplasia and no contrast enhancement (median survival, 11 years). Grade B indicates the presence of endothelial hyperplasia and/or contrast enhancement (median survival, 3.5 years). Five-year survival rates were 89% for Grade A and 60% for Grade B. The authors contend that delayed angiogenesis heralds the progression of an indolent type III pattern into a more aggressive tumor and hence shorter survival. Interestingly, the degree of nuclear atypia, mitosis, and necrosis did not correlate with survival.
The most common clinical manifestation of patients with oligodendrogliomas is seizures. Even when fixed neurological signs are evident, almost all patients experience seizures before the other signs develop. These tumors are best diagnosed with MR imaging. The most common finding is a hyperintensity on T2-weighted images. The cortex, where some authors believe oligodendrogliomas arise, is frequently involved.16 As noted, some tumors enhance after contrast administration. Most tumors are peripheral, and many occupy more than one lobe. The frontal lobe is the cortical area most often involved. A smaller number of tumors are deeper; tumors in children tend to develop in the thalamus.16
Like the treatment of low-grade astrocytomas, the treatment of oligodendrogliomas remains controversial. Surgical resection and radiation are often employed, and the role of chemotherapy has been increasing.
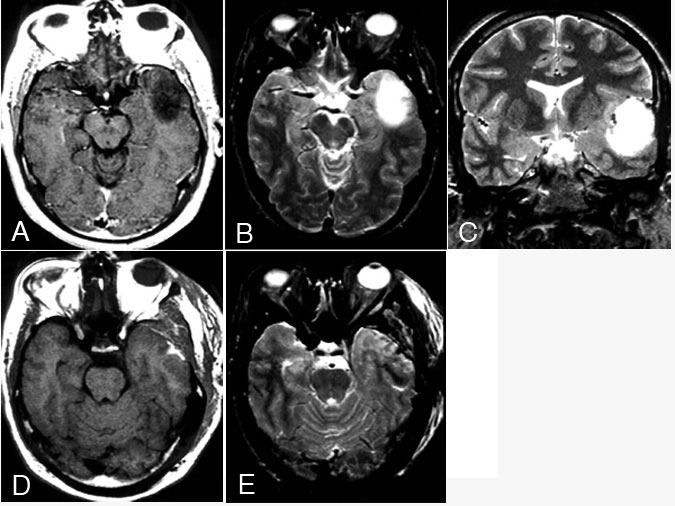
Most neurosurgeons, neuro-oncologists, and radiation oncologists recommend surgical resection as the initial treatment of oligodendroglioma, if possible. If the tumors are “completely” resected, such patients typically do well (Fig. 2). Radiation therapy is often added, especially for incomplete resections. Celli et al.11 reported 105 patients who underwent surgery. Their median survival was 64 months, and their 5-year survival rate was 52.4%. A more “benign” pathology was associated with longer survivals. Interestingly, patients who presented with seizure and no neurological signs did exceptionally well, with or without radiation (median survival, 122 months without radiation, 85 months with radiation). Radiation therapy helped those with mass effect and neurological signs. These results relating outcome to clinical presentation correspond to those of Daumas-Duport et al.15 for “pure” oligodendrogliomas.
Outcomes from similar retrospective studies have varied. Lindegaard et al.38 found that 108 irradiated patients had a significantly better median postoperative survival time (38 months) than 62 patients who did not receive radiotherapy (26.5 months, p = 0.039). Radiation therapy was most beneficial for patients with subtotal resections; its value was less clear in patients whose tumors were thought to have been totally resected. Shaw et al.69 also found that patients who underwent subtotal resections and radiotherapy lived longer than those undergoing subtotal resection alone. This series, however, was retrospective and patients often had not been treated with radiation. Gannett et al.19 found radiation therapy was associated with better survival rates, as did Allison et al.2
Bullard et al.,7 however, found no statistical difference in survival rates between 37 patients treated with radiation therapy and 34 patients who underwent surgery alone. Similarly, in a retrospective review of 82 cases, Kros et al.34 found no beneficial effect of radiation therapy on survival rate. Nor could Nijjar et al.49 demonstrate an effect of radiation therapy although only 10 of 72 patients received irradiation in their study.
Krouwer et al.35 reported a clinicopathological study of 52 patients with oligoastrocytomas, defined as having at least 10% neoplastic astrocytes and 10% neoplastic oligodendrocytes. The median survival was 75 weeks, and the 5-year survival rate was 29%. St. Anne-Mayo grade 2 patients had a median survival of 305 weeks; grade 3 patients, 217 weeks; and grade 4 patients, 55 weeks. Reviewing 71 patients with oligoastrocytomas, Shaw et al.68 noted that tumor grade (Kernohan) was strongly associated with survival. The median survival of 60 patients with grade 1 and 2 tumors was 6.3 years, and their 5- and 10-year survival rates were 58% and 32%, respectively. In contrast, the median survival of 11 patients with grade 3 and 4 tumors was 2.8 years (5- and 10-year survival rates of 36 and 9%, respectively). Age younger than 37 years, gross total resection, partial brain radiation, and radiation doses ³5,000 cGy were associated with longer survival times.
Finally, increasing evidence suggests that oligodendrogliomas and oligoastrocytomas may be sensitive to chemotherapy, especially if the tumors are anaplastic. Cairncross et al.10 treated 33 patients with either newly diagnosed or recurrent anaplastic oligodendrogliomas with a combination chemotherapy that consisted of procarbazine, CCNU, and PCV. Of the 24 eligible patients, 18 responded (nine completely) to the therapy. Kim et al.28 treated 32 patients with “grade III and grade IV oligoastrocytomas” using PCV chemotherapy. Ten patients had a complete response, and 19 had a partial response. Several other controlled trials of chemotherapy are now underway.
Conclusions
Primary brain tumors, especially gliomas, represent an especially difficult challenge to therapists for several reasons. First, the brain is unforgiving in its response to both tumor and therapy. Second, the tumor cells themselves are unstable and readily develop a resistance to treatment. Finally, the tumor’s environment is far removed from the therapist’s tools. Advances in understanding the biology of both tumor and normal brain cells promise to lead to new therapeutic approaches. Effective therapy, however, will likely continue to require a multimodality approach.
For now, glioblastomas multiforme and anaplastic gliomas should be treated with surgical resection, radiation therapy, and chemotherapy. Low-grade astrocytomas and oligodendrogliomas should be treated by resection and radiation therapy if there is substantial residual tumor or if the tumor has recurred after a previous resection. Chemotherapy is probably useful in the treatment of anaplastic oligodendrogliomas. Patients with these tumors should be referred for entry into clinical trials. Only by continued research will progress in treatment be made.
Acknowledgment
Photographs courtesy of Dr. Joseph Heiserman, Division of Neuroradiology, Barrow Neurological Institute.
References
- Albert FK, Forsting M, Sartor K, et al: Early postoperative magnetic resonance imaging after resection of malignant glioma: Objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 34:45-60, 1994
- Allison RR, Schulsinger A, Vogtama V, et al: Radiation and chemotherapy to improve outcome in oligodendroglioma. Int J Radiat Oncol Biol Phys 37:399-403, 1997
- Ameri A, Poisson M, Chauveinc L, et al: Treatment of recurrent malignant supratentorial gliomas with the association of carboplatin and etoposide: A phase II study. J Neurooncol 32:155-160, 1997
- Berger MS, Deliganis AV, Dobbins J, et al: The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer 74:1784-1791, 1994
- Black KL, Cloughesy T, Huang SC, et al: Intracarotid infusion of RMP-7, a bradykinin analog, and transport of gallium-68 ethylenediamine tetraacetic acid into human gliomas. J Neurosurg 86:603-609, 1997
- Brem H, Piantadosi S, Burger PC, et al: Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-Brain Tumor Treatment Group. Lancet 345:1008-1012, 1995
- Bullard DE, Rawlings CEI, Phillips B, et al: Oligodendroglioma: An analysis of the value of radiation therapy. Cancer 60:2179-2188, 1987
- Burger PC, Rawlings CE, Cox EB, et al: Clinicopathological correlations in the oligodendroglioma. Cancer 59:1345-1352, 1987
- Burger PC, Scheithauer BW, Vogel FS: Surgical Pathology of the Nervous System and its Coverings. New York, Churchill Livingstone: 1991
- Cairncross G, Macdonald D, Ludwin S, et al: Chemotherapy for anaplastic oligodendroglioma. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 12:2013-2021, 1994
- Celli P, Nofrone I, Palma L, et al: Cerebral oligodendroglioma: Prognostic factors and life history. Neurosurgery 35:1018-1035, 1994
- Couldwell WT, Hinton DR, Surnock AA, et al: Treatment of recurrent malignant gliomas with chronic oral high-dose tamoxifen. Clin Cancer Res 2:619-622, 1996
- Curran WJJ, Scott CB, Weinstein AS, et al: Survival comparison of radiosurgery-eligible and
-ineligible malignant glioma patients treated with hyperfractionated radiation therapy and carmustine: A report of Radiation Therapy Oncology Group 83-02. J Clin Oncol 11:857-862, 1993 - Daumas-Duport C: Histological grading of gliomas. Curr Opin Neurol Neurosurg 5:924-931, 1992
- Daumas-Duport C, Tucker ML, Kolles H, et al: Oligodendrogliomas. Part II: A new grading system based on morphological and imaging criteria. J Neurooncol 34:61-78, 1997
- Daumas-Duport C, Varlet P, Tucker ML, et al: Oligodendrogliomas. Part I: Patterns of growth, histological diagnosis, clinical and imaging correlations: A study of 153 cases. J Neurooncol 34:37-59, 1997
- Deutsch M, Green SB, Strike TA, et al: Results of a randomized trial comparing BCNU plus radiotherapy, streptozotocin plus radiotherapy, BCNU plus hyperfractionated radiotherapy, and BCNU following misonidazole plus radiotherapy in the postoperative treatment of malignant glioma. Int J Radiat Oncol Biol Phys 16:1389-1396, 1989
- Freeman SM, Abboud CN, Whartenby KA, et al: The “bystander effect:” Tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res 53:5274-5283, 1993
- Gannett DE, Wisbeck WM, Silbergeld DL, et al: The role of postoperative irradiation in the treatment of oligodendroglioma. Int J Radiat Oncol Biol Phys 30:567-573, 1994
- Glantz MJ, Burger PC, Herndon JE, et al: Influence of the type of surgery on the histologic diagnosis in patients with anaplastic gliomas. Neurology 41:1741-1744, 1991
- Glantz MJ, Choy H, Kearns CM, et al: Phase I study of weekly outpatient paclitaxel and concurrent cranial irradiation in adults with astrocytomas. J Clin Oncol 14:600-609, 1996
- Green SB, Byar DP, Walker MD, et al: Comparisons of carmustine, procarbazine, and high-dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treat Rep 67:121-132, 1983
- Greig NH, Ries LG, Yancik R, et al: Increasing annual incidence of primary malignant brain tumors in the elderly. J Natl Cancer Inst 82:1621-1624, 1990
- Gutin PH, Leibel SA, Wara WM, et al: Recurrent malignant gliomas: Survival following interstitial brachytherapy with high-activity iodine-125 sources. J Neurosurg 67:864-873, 1987
- Hiesiger EM, Green SB, Shapiro WR, et al: Results of a randomized trial comparing intra-arterial cisplatin and intravenous PCNU for the treatment of primary brain tumors in adults: Brain Tumor Cooperative Group Trial 8420A. J Neurooncol 25:143-154, 1995
- Karim ABMF, Maat B, Hatlevoll R, et al: A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) study 22844. Int J Radiat Oncol Biol Phys 36:549-556, 1996
- Kelly PJ, Hunt C: The limited value of cytoreductive surgery in elderly patients with malignant gliomas. Neurosurgery 34:62-66, 1994
- Kim L, Hochberg FH, Thornton AF, et al: Procarbazine, lomustine, and vincristine (PCV) chemotherapy for Grade III and Grade IV oligoastrocytomas. J Neurosurg 85:602-607, 1996
- Kleihues P, Burger PC, Scheithauer BW: Histological Typing of Tumours of the Central Nervous System. Geneva: World Health Organization, Springer-Verlag, 1993
- Knisely JPS, Haffty BG, Christopher SR: Early vs. delayed radiotherapy in a small cohort of patients with supratentorial low grade glioma. J Neurooncol 34:23-29, 1997
- Kondziolka D, Lunsford LD, Martinez AJ: Unreliability of contemporary neurodiagnostic imaging in evaluating suspected adult supratentorial (low-grade) astrocytoma. J Neurosurg 79:533-536, 1993
- Kreth FW, Faist M, Rossner R, et al: The risk of interstitial radiotherapy of low-grade gliomas. Radiother Oncol 43:253-260, 1997
- Kreth FW, Faist M, Rossner R, et al: Supratentorial World Health Organization Grade 2 astrocytomas and oligoastrocytomas. A new pattern of prognostic factors. Cancer 79:370-379, 1997
- Kros JM, Pieterman H, Van Eden CG, et al: Oligodendroglioma: The Rotterdam-Dijkzigt experience. Neurosurgery 34:959-966, 1994
- Krouwer HG, Van Duinen SG, Kamphorst W, et al: Oligoastrocytomas: A clinicopathological study of 52 cases. J Neurooncol 33:223-238, 1997
- Laws ER, Taylor WF, Clifton MB, et al: Neurosurgical management of low-grade astrocytoma of the cerebral hemispheres. J Neurosurg 61:665-673, 1984
- Leighton C, Fisher B, Bauman G, et al: Supratentorial low-grade glioma in adults: An analysis of prognostic factors and timing of radiation. J Clin Oncol 15:1294-1301, 1997
- Lindegaard K, Mork SJ, Eide GE, et al: Statistical analysis of clinicopathological features, radiotherapy, and survival in 170 cases of oligodendroglioma. J Neurosurg 67:224-230, 1987
- Loeffler JS, Alexander E, III, Shea WM, et al: Radiosurgery as part of the initial management of patients with malignant gliomas. J Clin Oncol 10:1379-1385, 1992
- Loeffler JS, Shrieve DC, Alexander E, III: Radiosurgery for glioblastoma multiforme: The importance of selection criteria. Int J Radiat Oncol Biol Phys 30:731-733, 1994
- Ludwig CL, Smith MT, Godfrey AD, et al: A clinicopathological study of 323 patients with oligodendrogliomas. Ann Neurol 19:15-21, 1986
- Lunsford LD, Somaza S, Kondziolka D, et al: Survival after stereotactic biopsy and irradiation of cerebral nonanaplastic, nonpilocytic astrocytoma. J Neurosurg 82:523-529, 1995
- Mahaley MS: Neuro-oncology index and review (adult primary brain tumors). Radiotherapy, chemotherapy, immunotherapy, photodynamic therapy. J Neurooncol 11:85-147, 1991
- Mahaley MSJ, Mettlin C, Natarajan N, et al: National survey of patterns of care for brain-tumor patients. J Neurosurg 71:826-836, 1989
- Malkin MG, Mason WP, Lieberman FS, et al: Phase I study of SU101, a novel signal transduction inhibitor, in recurrent malignant glioma (abstract). Proc Am Assoc Clin Oncol 16:385a, 1997
- Mehta MP, Masciopinto J, Rozental J, et al: Stereotactic radiosurgery for glioblastoma multiforme: Report of a prospective study evaluating prognostic factors and analyzing long-term survival advantage. Int J Radiat Oncol Biol Phys 30:541-549, 1994
- Nazzaro JM, Neuwelt EA: The role of surgery in the management of supratentorial intermediate and high-grade astrocytomas in adults. J Neurosurg 73:331-344, 1990
- Nicolato A, Gerosa MA, Fina P, et al: Prognostic factors in low-grade supratentorial astrocytomas: A uni-multivariate statistical analysis in 76 surgically treated adult patients. Surg Neurol 44:208-223, 1995
- Nijjar TS, Simpson WJ, Gadalla T, et al: Oligodendroglioma: The Princess Margaret Hospital experience (1958-1984). Cancer 71:4002-4006, 1993
- North CA, North RB, Epstein JA, et al: Low-grade cerebral astrocytoma: Survival and quality of life after radiation therapy. Cancer 66:6-14, 1990
- Peterson K, Cairncross JG: Oligodendroglioma. Cancer Invest 14:243-251, 1996
- Phillippon JH, Clemenceau SH, Fauchon FH, et al: Supratentorial low-grade astrocytomas in adults. Neurosurgery 32:554-559, 1993
- Piepmeier J, Christopher S, Spencer D, et al: Variations in the natural history and survival of patients with supratentorial low-grade astrocytomas. Neurosurgery 38:872-878, 1996
- Piepmeier JM, Christopher S: Low-grade gliomas: Introduction and overview. J Neurooncol 34:1-3, 1997
- Prados MD, Schold SC, Spence AM, et al: Phase II study of paclitaxel in patients with recurrent malignant glioma. J Clin Oncol 14:2316-2321, 1996
- Ram Z, Culver KW, Walbridge S, et al: Toxicity studies of retroviral-mediated gene transfer for the treatment of brain tumors. J Neurosurg 79:400-407, 1993
- Recht LD, Lew R, Smith TW: Suspected low-grade glioma: Is deferring treatment safe? Ann Neurol 31:431-436, 1992
- Riggs JE: Rising primary malignant brain tumor mortality in the elderly: A manifestation of differential survival. Arch Neurol 52:571-575, 1995
- Rosenblum MK, Delattre JY, Walker RW, et al: Fatal necrotizing encephalopathy complicating treatment of malignant gliomas with intra-arterial BCNU and irradiation: A pathological study. J Neurooncol 7:269-281, 1989
- Scerrati M, Roseli R, Iacoangeli M, et al: Prognostic factors in low grade (WHO grade II) gliomas of the cerebral hemispheres: The role of surgery. J Neurol Neurosurg Psychiatry 61:291-296, 1996
- Shapiro WR: Treatment of neuroectodermal brain tumors. Ann Neurol 12:231-237, 1982
- Shapiro WR: Therapy of adult malignant brain tumors: What have the clinical trials taught us? Semin Oncol 13:38-45, 1986
- Shapiro WR, Green SB, Burger PC, et al: Randomized trial of three chemotherapy regimens and two radiotherapy regimens in postoperative treatment of malignant glioma: Brain Tumor Cooperative Group Trial 8001. J Neurosurg 71:1-9, 1989
- Shapiro WR, Green SB, Burger PC, et al: A randomized comparison of intra-arterial versus intravenous BCNU, with or without intravenous 5-fluorouracil, for newly diagnosed patients with malignant glioma. J Neurosurg 76:772-781, 1992
- Shapiro WR, Shapiro JR, Walker RW: Management of specific malignancies: Central nervous system, in Abeloff MD, Armitage JO, Lichter AS, et al (eds): Clinical Oncology. New York: Churchill Livingstone, 1995, pp 851-912
- Shaw EG, Daumas-Duport C, Scheithauer BW, et al: Radiation therapy in the management of low-grade supratentorial astrocytomas. J Neurosurg 70:853-861, 1989
- Shaw EG, Scheithauer BW, O’Fallon JR: Supratentorial gliomas: A comparative study by grade and histologic type. J Neurooncol 31:273-278, 1997
- Shaw EG, Scheithauer BW, O’Fallon JR, et al: Mixed oligoastrocytomas: A survival and prognostic factor analysis. Neurosurgery 34:577-582, 1994
- haw EG, Scheithauer BW, O’Fallon JR, et al: Oligodendrogliomas: The Mayo Clinic experience. J Neurosurg 76:428-434, 1992
- Shibamoto Y, Kitakabu Y, Takahashi M, et al: Supratentorial low-grade astrocytoma: Correlation of computed tomography findings with effect of radiation therapy and prognostic variables. Cancer 72:190-195, 1993
- Simpson JR, Horton J, Scott C, et al: Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: Results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys 26:239-244, 1993
- Slotman BJ, Kralendonk JH, Van Alphen HAM, et al: Hypofractionated radiation therapy in patients with glioblastoma multiforme: Results of treatment and impact of prognostic factors. Int J Radiat Oncol Biol Phys 34:895-898, 1996
- Smith MT, Ludwig CL, Godfrey AD, et al: Grading of oligodendrogliomas. Cancer 52:2107-2114, 1983
- Soffietti R, Chio A, Giordana MT, et al: Prognostic factors in well-differentiated cerebral astrocytomas in the adult. Neurosurgery 24:686-692, 1989
- Taki T, Ohnishi T, Arita N, et al: Anti-proliferative effects of TNP-470 on human malignant glioma in vivo: Potent inhibition of tumor angiogenesis. J Neurooncol 19:251-258, 1994
- Taphoorn MJB, Schiphorst AK, Snoek FJ, et al: Cognitive functions and quality of life in patients with low-grade gliomas: The impact of radiotherapy. Ann Neurol 36:48-54, 1994
- Vecht CJ: Effect of age on treatment decisions in low-grade glioma. J Neurol Neurosurg Psychiatry 56:1259-1264, 1993
- Vertosick FT, Selker RG, Arena VC: Survival of patients with well-differentiated astrocytomas diagnosed in the era of computed tomography. Neurosurgery 28:496-501, 1991
- Vertosick FT, Selker RG, Pollack IF, et al: The treatment of intracranial malignant gliomas using orally administered tamoxifen therapy: Preliminary results in a series of “failed” patients. Neurosurgery 30:897-903, 1992
- Walker MD, Alexander E, Hunt WE, et al: Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas: A cooperative clinical trial. J Neurosurg 49:333-343, 1978
- Walker MD, Green SB, Byar DP, et al: Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. N Engl J Med 303:1323-1329, 1980
- Walker MD, Strike TA, Sheline GE: An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys 5:1725-1731, 1979
- Werner MH, Phuphanich S, Lyman GH: The increasing incidence of malignant gliomas and primary central nervous system lymphoma in the elderly. Cancer 76:1634-1642, 1995
- Winger MJ, Macdonald DR, Cairncross JG: Supratentorial anaplastic gliomas in adults. The prognostic importance of extent of resection and prior low-grade glioma. J Neurosurg 71:487-493, 1989
- Wood JR, Green SB, Shapiro WR: The prognostic importance of tumor size in malignant gliomas: A computed tomographic scan study by the Brain Tumor Cooperative Group. J Clin Oncol 6:338-343, 1988
