
Application of Frameless Stereotaxy to Spinal Surgery
Juan Bartolomei, MD
Jeffrey S. Henn, MD
G. Michael Lemole, Jr., MD
James Lynch, MD
Curtis A. Dickman, MD
Volker K.H. Sonntag, MD
Division of Neurological Surgery, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
Frameless stereotaxy has several potential applications in spinal surgery. The technique offers precise anatomical localization, which can improve placement of instrumentation or localization of lesions. Compared with cranial frameless stereotaxy, several considerations are unique to the spine. This article discusses the applications and limitations of spinal frameless stereotaxy and considers potential future directions.
Key Words: frameless navigational systems, image-guided surgery, minimally invasive surgery, spinal surgery
Frameless stereotactic image-guided surgery has been widely and successfully applied to intracranial procedures. This technology has more recently gained popularity among spinal surgeons. The most common application of frameless stereotaxy to spinal surgery is in the placement of instrumentation. Frameless stereotaxy also has potential application in the surgical treatment of spinal tumors and vascular malformations. This article considers the advantages and potential limitations of image-guided spinal surgery, the available navigational systems, and clinical applications of the technology.
Image Guidance and Spinal Surgery
Image-guidance techniques for the spine differ in many ways from cranial surgery. First, the spine is a relatively mobile structure, and significant movement occurs intraoperatively due to respiratory excursion and changes in patient positioning. Consequently, a reference frame must be applied directly to an adjacent vertebral segment to track positional changes during surgery. Second, although cranial frameless stereotaxy relies on registration using scalp fiducial markers, registration for spinal image-guidance relies on anatomical landmarks within the spine itself. Third, the spine is composed of several distinct, rigid anatomical structures that can change relative position as a result of operative manipulation. Compared to cranial procedures, these differences make the use of stereotactic image-guided techniques in the spine more challenging.
Spinal anatomy does provide some advantages with regard to image- guided surgery. Although the relationship between vertebrae may change between imaging and patient positioning, the anatomy within each vertebra remains constant. That is, the relationships among the spinous process, transverse process, and pedicle of a given vertebra remain stable regardless of intraoperative deformation of the spinal column. New algorithms that can merge intraoperative fluoroscopy with preoperative imaging (computed tomography [CT] and magnetic resonance [MR] imaging) are being developed. The anatomy of each vertebral body and its relationship with the other adjacent bodies are integrated to reflect the new position of the spine. Therefore, an updated real- time image of the spine can be obtained. Using these algorithms, deformational changes of the spine can be accounted for and are generally less of a problem than the similar problem of brain shift during intracranial frameless stereotaxy.
To perform registration, the surface of the spine must be exposed surgically. Once the spine is exposed, registration is possible. An intrinsic advantage of spinal anatomy is that landmarks can be reregistered at any time during the procedure if registration is lost.
Role of Image Guidance in Spinal Instrumentation
An understanding of the anatomical relationships of the spine is critical for the safe and successful placement of instrumentation. The increased use of instrumentation for the spine and the risks of inadvertent neural or vascular injury make frameless stereotaxy a valuable surgical adjunct. Before the advent of image-guided surgery, accurate placement and insertion of spinal instrumentation relied solely on the surgeon’s skill and ability to infer three-dimensional anatomy from intraoperative radiographs.
The placement of pedicle screws relies on understanding the anatomical relationships of the pedicle, pars inter articularis, and transverse process. Significant muscle retraction and bony decompression are often necessary to identify these anatomical landmarks. The resultant exposure enables a combination of visual cues, direct physical palpation, and fluoroscopic guidance to be used to place pedicle screws. Failure to understand the three-dimensional anatomy of the bony relationships leads to an unacceptable level of morbidity. Poor quality intraoperative imaging, unusual body habitus, or the presence of underlying rotational deformities can also contribute to this problem.
Several studies have assessed the value of fluoroscopy and anatomical landmarks in assuring accurate placement of instrumentation. Using standard surgical approaches to place pedicle screws, Weinstein et al.[14] reported that the incidence of broken pedicles was 21%. In an anatomical study, Heller et al.[8] inserted cervical lateral mass screws using the Magerl and the Roy-Camille techniques. The rate of cervical nerve root injury using the Magerl technique was 7.3%, and the rate of facet violation using the Roy-Camille technique was 22.5%. These studies demonstrate the limitation of traditional instrumentation techniques.
Frameless stereotaxy allows precise localization of anatomic structures and can be a valuable adjunct in the placement of spinal instrumentation. The planned trajectory of instruments can be visualized, potentially improving the precision and safety of the procedure. Stereotactic guidance in the spine has been shown to be useful for placing pedicle screws in the cervical, thoracic, and lumbar regions.[3,4] Frameless stereotaxy has also been used to place C1-C2 transarticular screws, to define the trajectory of screws, and to minimize the risk of injuring the vertebral artery.[16]
Role of Image Guidance in Lesion Localization
Frameless stereotaxy can be useful for precise localization of tumors, vascular malformations, and pathological anatomy of the spine. Although these lesions can be localized in the traditional fashion by using anatomic landmarks, frameless stereotaxy offers several potential advantages: precise lesion localization, improved understanding of regional neurovascular anatomy, and feedback about the extent of lesion resection.
Frameless stereotaxy is most useful when a lesion cannot be readily identified based on surface anatomy. Examples include primary bone tumors, intramedullary tumors, and spinal cord cavernous malformations. Once the lesion has been localized accurately, frameless stereotaxy also provides valuable feedback about the extent of resection. Finally, by defining regional anatomy, frameless stereotaxy can be particularly useful for tumors or vascular malformations adjacent to critical structures. For example, a large cervical nerve sheath tumor involving the vertebral artery can be resected more safely with the use of frameless stereotaxy than without. Finally, by accurately delineating pathological anatomy, frameless stereotaxy can provide localization when standard anatomical cues are distorted, for example, during a transoral resection of a degenerative odontoid process for anterior decompression of the cervicomedullary junction.[15]

Spinal Navigation Systems
Several image-guided stereotactic systems are available commercially for intracranial or spinal surgery. Although the basic concept underlying these systems is similar, their methods for tracking and performing intraoperative neuronavigation differ. Common tracking devices used in these systems include light-emitting diodes (LEDs), articulated arms, and magnetic field sources.
Probably the most widely used systems in spinal surgery rely on LED tracking. These systems consist of an infrared camera that serves as a tracking device. The camera localizes the position of instruments in space and provides this information to the computer. Other systems use an articular arm that incorporates a multijoint probe that provides mechanical localization of points in space. Magnetic field-based systems are similar to the LED systems except that the source for tracking instruments is a magnetic field that tracks the position of surgical instruments through small receiving antennae at the tip of the instruments.
We have primarily used the StealthStation LED infrared-based system (Medtronic Surgical Navigation Technologies, Louisville, CO; Fig. 1), which is divided into three components. The first component is an infrared camera that either receives emitted infrared signals from the LED array in the surgical instruments (active system) or emits an infrared light that is reflected back to the camera by the surgical instruments in the field (passive system). In the active system, the instruments in the surgical field transmit infrared signals from LED arrays. Passive systems use only reflecting elements and consequently provide more freedom during surgical navigation than active systems.
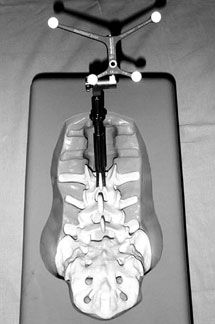
The second component is a reference-frame tracking device attached to the spinous process with a bone clamp (Fig. 2). Alternatively, some systems incorporate the tracking device into the retractors. The tracking device provides feedback to the camera about the position of the spine during surgery. This feedback is needed to compensate for motion of the spine in response to mechanical ventilation when the patient is placed prone. Using the tracking device, the motion of the spine is detected and quantified by the camera array in real-time, thereby enhancing the accuracy of the registration process and navigation throughout surgery. This real-time tracking technique is known as dynamic referencing.[6]
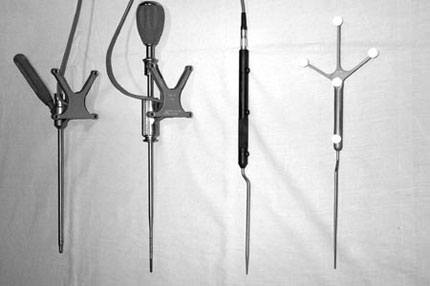
The final components of the LED infrared-based system are the actual surgical tools (i.e., pointers, awls, drill guides, pedicle finders; Fig. 3). These tools have built-in LED arrays that allow the camera to track their position in space relative to the tracking device.
A relatively new technique in image-guided spinal surgery is the use of registration based on intraoperative fluoroscopy. An example is the FluoroNav system (Medtronics, Louisville, CO), which can be used to place pedicle screws. After exposure is completed and a tracking device has been applied, intraoperative fluoroscopic images are obtained using a special overlay registration grid. These fluoroscopic images are then used for image-based navigation. The potential benefits include a decreased need for repeat fluoroscopic imaging and the ability to view anteroposterior and lateral images simultaneously during pedicle screw placement.[12]
Techniques of Spinal Navigation
The first step in image-guided surgery is the acquisition of either CT or MR images. In spinal surgery, CT is usually the best diagnostic modality because of its superior demonstration of bone anatomy. In the case of spinal tumors, either CT or MR imaging may be used depending on the specifics of the case. In general, thin cuts (usually 1 mm) are obtained through the levels of interest. Typically, surface fiducial markers are not used for image-guided spinal surgery and are not applied. Exceptions include transoral procedures or lesions in the upper cervical spine, when cranial fiducial markers may be useful.
Once the images are obtained, they are transferred to the computer workstation through tape, CD, or a hospital-based network. The system’s computer software allows the image data to be displayed using either standard two-dimensional planes or three- dimensional reconstruction.
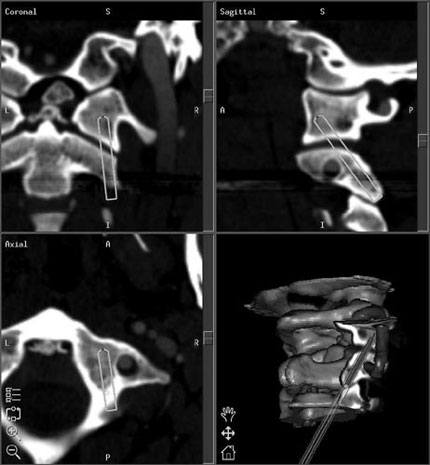
During preoperative planning, specific points within the spine are identified as potential registration sites using the three-dimensional reformatted images. Examples of these points include the tips of the spinous processes, the superior or inferior aspects of the spinous processes, the midpoint of the pars interarticularis, or the superior and inferior aspects of the transverse processes. Occasionally, it is also useful to plan the trajectories for placing instrumentation (Fig. 4). Some systems include software-based virtual instrumentation that can be placed and viewed on the reconstructed three-dimensional images.
After the preoperative preparation, the patient is taken to the operating room and positioned in a routine fashion. The workstation containing the computer and video display is routinely positioned opposite the surgeon to allow a direct view of the monitor. Positioning of the infrared camera is crucial. It must be placed directly in the line of sight of the instrument that is being tracked. Usually, the best location for the camera is at the foot or head of the operating table. The camera must be adjusted if the line of sight between the camera and the instruments is obscured.
After a routine surgical exposure is performed, the tracking device is clamped onto a spinous process. Ideally, the tracker should be placed as close as possible to the planned site of intervention to minimize errors of registration and navigation. Once the tracking device has been securely attached to the bone, registration can proceed.
During registration, the surgeon must match exposed surface landmarks on the patient with the pre selected registration sites based on the three-dimensional image reconstructions. The process of registration is relatively straightforward and rapid. At least four points of registration are necessary. Using more points and increasing the separation between the points increases the accuracy of registration. To avoid loss of registration, excessive forces, spinal motion, or movement of the tracking device must be avoided. Consequently, placement of the tracking device and registration are performed after exposure and bony decompression. Of course, registration must precede removal of bone registration landmarks.
After each point has been identified and matched with its corresponding point in the imaging sequence, the computer can calculate the transformation and resulting registration. Accuracy can also be improved by augmenting the paired-point registration with a surface-based registration. This process involves touching multiple points on the surface of the spine to create a three-dimensional contour of the structure. Typically, this process requires as many as 30 to 40 points. Based on this contour, the computer then matches the surface points with computer-rendered surfaces in the imaging data. The combination of paired-point and surface-based registration can significantly decrease errors during registration and thereby improve accuracy. During the registration process, the computer can determine the mean error of registration and can help identify specific points that are inaccurate. A point that is associated with a relatively large error may be removed to increase the overall accuracy of registration.
Drilling small holes at the locations of the preselected registration points can also be useful if reregistration is necessary during surgery. Of course, these holes must be placed on structures that will remain after decompression.
The accuracy of registration in spinal surgery can be increased with other strategies, which include the use of percutaneously placed spinal markers with intraoperative CT, percutaneous ultrasonography, or both.[17] A two-stage procedure, in which the spine is exposed and internal fiducials are placed during the first stage, is another option. The wound is then closed, and the patient undergoes imaging with more accurate registration during the second stage.[13] Although these techniques are complicated, accurate registration is sometimes paramount. These techniques are particularly useful for patients with significantly deformed anatomical landmarks that make registration and navigation harder to achieve.
Once registration is completed, navigation can proceed. A pointing probe is used to verify that the surgical anatomy matches well with the anatomy displayed on the computer monitor. The anatomy must be correlated several times throughout the procedure to verify that registration has been maintained. This correlation should also be performed at several different sites because the accuracy of registration may be a function of the distance from the tracking device.
Current and Future Clinical Applications
There are several important applications for image-guided surgery in the spine. Typical examples include pedicle screw fixation, placement of C1-C2 transarticular screws, and the resection of certain spinal tumors and vascular malformations. Frameless stereotaxy is particularly useful for resecting spinal tumors when the bony anatomy is distorted and the standard visual references are lost.[15] Image-guided navigation can assist with the identification and safe resection of spinal tumors.
Application of image-guided navigation to the placement of C1-2 trans articular screws has also been described.[16] In this procedure, there is a significant risk of vertebral artery injury if the trajectory of the screws is not carefully planned. Some systems allow virtual screws to be placed to determine the length, diameter, and trajectory of the transarticular screws.
Placement of pedicle screws in the thoracic and lumbar spine has also been aided by image-guided navigation.[5,11] Compared to conventional techniques, most studies report that frameless stereotactic guidance improves the accuracy of screw placement and minimizes the chance of nerve injury.
Another potential application of image-guided navigation is the placement of cervical pedicle screws (i.e., to treat traumatic injuries). In the case of unilateral or bilateral locked facets, several reports have demonstrated the benefits of traction and closed reduction followed by cervical pedicle screw fixation.[1,2] This posterior approach permits distraction and reduction of complex traumatic injuries and simultaneous surgical fixation. This technique avoids the need for a separate anterior decompression and fusion. In these cases, image-guided systems are very helpful to avoid injury to the vertebral artery or nerve root when pedicle screws are placed into relatively small pedicles from C3 to C6.
Potential future applications of image-guided spinal surgery include the ability to register transcutaneously, which could facilitate minimally invasive, percutaneous image-guided spinal surgery. These techniques might be used in conjunction with established endoscopic, anterior, or anterolateral approaches to the cervical spine[7,10] and with posterolateral lumbar discectomies.[9] Posterior approaches such as cervical foraminotomies for the treatment of unilateral radiculopathy also might be combined with endoscopy and image-guided systems. These developments reflect a general trend toward minimally invasive surgery.
Conclusion
Frameless stereotaxy has several potential applications in spinal surgery. The technique will not supplant the basic anatomical knowledge and skill that surgeons need to perform demanding and spinal procedures. It does, however, provide an added element of confidence, particularly when position and accuracy are critical. Like any new technique, incorporation of image-guided navigation into a surgeon’s armamentarium requires patience and dedication. However, the advantages of image-guided spinal surgery for appropriately selected cases are substantial.
References:
- Abumi K, Shono Y, Kotani Y, et al: Indirect posterior reduction and fusion of the traumatic disc by using a cervical pedicle screw system. J Neurosurg (Spine) 92:30-37, 2000
- Albert TJ, Klein GR, Joffe D, et al: Use of cervico thoracic junction pedicle screws for reconstruction of complex cervical spine pathology. Spine 23:1596-1599, 1998
- Bolger C, Wigfield C: Image-guided surgery: Applications to the cervical and thoracic spine and a review of the first 120 procedures. J Neurosurg (Spine) 92:175-180, 2000
- Bolger C, Wigfield C, Melkent T, et al: Frameless stereotaxy and anterior cervical surgery. Comput Aided Surg 4:322-327, 1999
- Choi WW, Green BA, Levi AD: Computer-assisted fluoroscopic targeting system for pedicle screw insertion. Neurosurgery 47:872-878, 2000
- Foley KT, Smith MM: Image-guided spine surgery. Neurosurg Clin N Am 7:171-186, 1996
- Fontanella A: Endoscopic microsurgery in herniated cervical discs. Neurol Res 21:31-38, 1999
- Heller JG, Carlson GD, Abitbol JJ, et al: Anatomic comparison of the Roy-Camille and Magerl techniques for screw placement in the lower cervical spine. Spine 16:S552-S557, 1991
- Hermantin FU, Peters T, Quartararo L, et al: A prospective, randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J Bone Joint Surg Am 81:958-965, 1999
- Jho HD: Decompression via microsurgical anterior foraminotomy for cervical spondylotic myelopathy. Technical note. J Neurosurg 86:297-302, 1997
- Kalfas IH, Kormos DW, Murphy MA, et al: Application of frameless stereotaxy to pedicle screw fixation of the spine. J Neurosurg 83:641-647, 1995
- Rampersaud YR, Foley KT, Shen AC, et al: Radiation exposure to the spine surgeon during fluoroscopically assisted pedicle screw insertion. Spine 25:2637-2645, 2000
- Salehi SA, Ondra SL: Use of internal fiducial markers in frameless stereotactic navigational systems during spinal surgery: Technical note. Neurosurgery 47:1460-1462, 2000
- Weinstein JN, Spratt KF, Spengler D, et al: Spinal pedicle fixation: Reliability and validity of roentgenogram-based assessment and surgical factors on successful screw placement. Spine 13:1012-1018, 1988
- Welch WC, Subach BR, Pollack IF, et al: Frameless stereotactic guidance for surgery of the upper cervical spine. Neurosurgery 40:958-963, 1997
- Weidner A, Wahler M, Chiu ST, et al: Modification of C1-C2 transarticular screw fixation by image-guided surgery. Spine 25:2668-2774, 2000
- Winkler D, Vitzthum HE, Seifert V: Spinal markers: A new method for increasing accuracy in spinal navigation. Comput Aided Surg 4:101-104, 1999
