
Pleomorphic Xanthoastrocytoma Presenting with Intraparenchymal and Subdural Hematoma: Case Report
Vivek R. Deshmukh, MD
Frank P. K. Hsu, MD
Jeffrey D. Klopfenstein, MD
Stephen W. Coons, MD*
Robert F. Spetzler, MD
Divisions of Neurological Surgery and *Neuropathology, Barrow Neurological Institute, St. Joseph’s Hospital and Medical Center, Phoenix, Arizona
Abstract
Pleomorphic xanthoastrocytomas (PXAs) are low-grade tumors of adolescents and young adults that typically manifest with seizures. We report the second patient who became symptomatic with a life-threatening intracranial hemorrhage. A 38-year-old woman experienced the acute onset of a severe headache and diminished level of consciousness. Computed tomography (CT) showed a large acute right intraparenchymal hematoma, acute subdural hematoma, and a rightto-left midline shift. She was lethargic but followed commands before surgery. Cerebral angiography ruled out a vascular malformation or cerebral aneurysm as the cause of hemorrhage. She underwent a right pterional craniotomy, evacuation of the hematoma, and gross total resection of a temporal tumor. Histological analysis identified a PXA. At discharge the patient was neurologically intact. PXAs can become symptomatic with intraparenchymal, subdural, and subarachnoid hemorrhage that manifests as an apoplectic event, presumably reflecting extensive meningeal involvement. The case demonstrates that this typically low-grade tumor can be associated with a life-threatening presentation.
Key Words: intraparenchymal hematoma, pleomorphic xanthoastrocytoma, subdural hematoma
Pleomorphic xanthoastrocytomas (PXAs) are low-grade tumors of adolescents and young adults and typically associated with a favorable prognosis. [7,8,14,15] They most often become symptomatic with seizures. We report a patient with a PXA who became symptomatic with a life-threatening intracranial hemorrhage. This report is the second case of a PXA manifesting with intracranial hemorrhage and the first in which the patient survived the event.[12]
Illustrative Case
A 38-year-old woman with a long history of headaches experienced the sudden onset of a right temporal headache the day before her admission. Her level of consciousness progressively declined, and she was evaluated and intubated at an outside hospital. Computed tomography (CT) showed a large right temporal intraparenchymal hemorrhage, an acute right frontotemporal subdural hematoma, and subarachnoid hemorrhage. She was transferred to our facility for further care. She had no history of hypertension, bleeding diathesis, trauma, or illicit drug use, and her medical history was unremarkable.
On examination the patient was lethargic. Her cranial nerve examination was normal. She sluggishly followed commands. Her diminished level of consciousness precluded a detailed motor and sensory assessment. She appeared to move her extremities symmetrically.
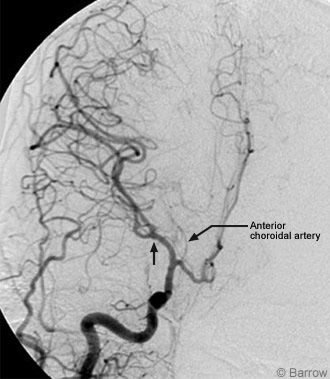
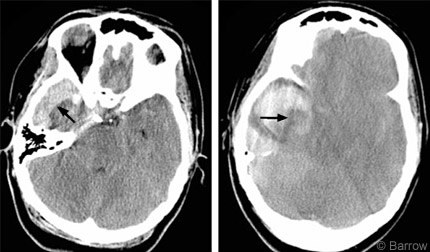
The large nontraumatic intracranial hemorrhage prompted an angiographic evaluation for vascular lesions, but the study was negative. The right common carotid artery injection demonstrated significant round shift of the middle cerebral artery candelabra and right-to left displacement of the anterior cerebral arteries (Fig. 1). Repeat CT demonstrated progression of the subdural hematoma and enlargement of the intraparenchymal hematoma (Fig. 2) compared to the outside diagnostic films. It also showed complete obliteration of the basal cisterns with imminent herniation.
A right pterional craniotomy was performed emergently. The subdural hematoma and intraparenchymal hematoma were evacuated. Upon removal of the hematoma, a nodular mass based on the floor of the temporal fossa was visible. The hematoma had surrounded this mass, which was resected with standard microsurgical techniques. The tumor itself was not vascular. No vascular injury was noted. The preliminary diagnosis was atypical meningioma. A drain was left in the subdural space.
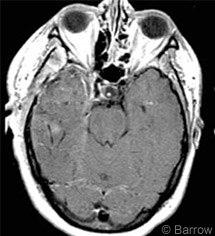
The patient was monitored in the intensive care unit for 2 days. Postoperative CT confirmed excellent evacuation of the hematoma and resolution of the midline shift. Postoperative magnetic resonance imaging (MRI) showed no residual tumor (Fig. 3). She recovered completely and was discharged home on postoperative Day 5. She was neurologically intact and had suffered no complications.
Histology
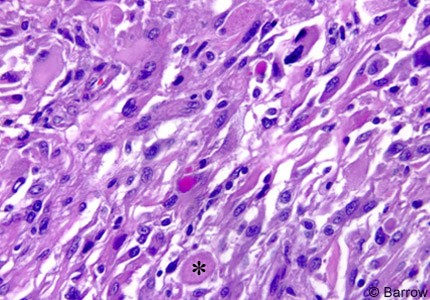
Microscopic examination demonstrated the typical features of PXA. Hypercellularity was marked. The preponderant cell population was composed of large fibrillary astrocytes associated with only mild-to-moderate atypia. Numerous cells with large, highly atypical nuclei scattered throughout the matrix often had abundant cytoplasm with a fibrillary, homogeneous “ground glass,” or vacuolated appearance. The latter two features reflected lipid accumulation. Numerous eosinophilic granular bodies were present. The tumor cells were immunoreactive for glial fibrillary acidic protein (GFAP),consistent with glial differentiation. Scattered cells were also reactive for the neuronal marker synaptophysin. No mitotic figures were present, and the MIB-1 labeling index was 1 to 3%, indicating modest proliferative activity. Neither microvascular proliferation nor tumor necrosis was present (Fig. 4).
Discussion
In 1979 PXAs were defined as a unique clinicopathologic entity.[10] Their ominous histological features, including cellular pleomorphism, nuclear atypia, and presence of multinucleate giant cells, belie their benign nature. The cell of origin is uniformly the astrocyte as demonstrated by their GFAP positivity. These tumors are usually located superficially in the temporal lobe and frequently abut if not involve the dura. PXAs have been reported within the posterior hypophysis, retina, pineal gland, cerebellum, and spinal cord.[1,9,14,20] MRI shows a cystic lesion with an enhancing mural nodule.[16] The radiographic differential diagnosis includes ganglioglioma and pilocytic astrocytoma.
This disease of adolescents and young adults usually manifests with seizures.[3,19] Less common symptoms and signs, which include headaches, nausea, vomiting, motor/sensory deficits, and papilledema, can be ascribed to increased intracranial pressure and regional mass effect. In fact, our patient had a long history of chronic daily headaches that had never been investigated until she sustained the massive, life-threatening hemorrhage.
The literature suggests that patients with PXA tend to have a favorable prognosis. In an early review of 35 patients with PXA, Whittle et al.19 noted almost a 50% survival rate 7.4 years after diagnosis. More recently, Pahapill et al.[15] reported an actuarial survival rate of 82% at 10 years. They found that the presence of necrosis portended a poor outcome. Fouladi et al.[6] reviewed 13 pediatric patients with PXA. Ten patients were alive at 41 months, and gross total resection prolonged disease control in 85%. Finally, Giannini et al.[7] described 71 patients with PXA who had an overall survival rate of 70% at 10 years. Mitotic activity and extent of resection predicted recurrence-free survival and overall survival. Necrosis correlated significantly with the overall survival rate but not with the recurrence-free survival rate. Several reports highlight the potential for malignant transformation of PXA and provide justification for routine radiographic follow up of these patients. [2,5,17]
Hemorrhage is either a rare presentation of this tumor or is underreported. Histologically, PXAs resemble moderately cellular astrocytic tumors and are associated with nuclear pleomorphism, multinucleated giant cells, and cystic areas containing proteinaceous fluid. The amount of reticulin-containing stroma is variable.13 PXAs are not associated with vascular endothelial proliferation. [7,13] These tumors are not typically hypervascular on microscopic examination. Why, then, did our patient suffer such a dramatic intracranial hemorrhage?
Lee et al.[11] described a single case of an arteriovenous malformation (AVM) associated with a PXA. The AVM was demonstrated on preoperative angiography. Our patient, however, had no AVM, and no portion of the histological specimen resembled the nidus of an AVM. Therefore this pathophysiology seems unlikely. Similar to hemorrhage within an arachnoid cyst, trauma can cause a vessel stretched by a tumor mass to tear. Our patient, however, had no history of trauma. An angiomatous variant of PXA with prominent vascularity has been described,[18] but microvascular proliferation was absent in our patient. PXAs have been known to invade the adjacent leptomeninges and dura.[13] Fouladi et al.[6] reported meningeal invasion in 12 of 13 patients with PXA. De Jesús et al.[4] described a recurrent PXA that invaded the tentorium and falx cerebri. Levy et al.[12] described a 46-year-old woman who developed a massive left temporal hematoma and subarachnoid hemorrhage from a PXA. She died despite evacuation of the hematoma and resection of the temporal PXA. They suggested that erosion of a cortical vessel may have been the underlying cause. In our case both the tumor mass and the subdural hematoma were primarily along the temporal floor. Ostensibly, the tumor eroded a vessel causing a hematoma that propagated to fill the cystic portion of the tumor and surrounded the mural nodule. Given the known predilection of these tumors for dural or leptomeningeal involvement, erosion of a cortical or dural vessel at the floor of the temporal fossa is the most rational explanation for our patient’s presentation.
Conclusion
PXAs are rare astrocytic tumors that tend to manifest with seizures. Clinicians, however, must be aware that these low-grade tumors also can become symptomatic with massive intracranial hemorrhage that can pose a life-threatening risk. Timely diagnosis and surgical treatment can yield rewarding clinical results.
References
- Arita K, Kurisu K, Tominaga A, et al: Intrasellar pleomorphic xanthoastrocytoma: Case report. Neurosurgery 51:1079-1082, 2002
- Chakrabarty A, Mitchell P, Bridges LR, et al: Malignant transformation in pleomorphic xanthoastrocytoma—
a report of two cases. Br J Neurosurg 13:516-519, 1999 - Davies KG, Maxwell RE, Seljeskog E, et al: Pleomorphic xanthoastrocytoma—report of four cases, with MRI scan appearances and literature review. Br J Neurosurg 8:681-689, 1994
- De Jesús O, Rifkinson N: Pleomorphic xanthoastrocytoma with invasion of the tentorium and falx. Surg Neurol 43:77-79, 1995
- de Tella OI Jr, Herculano MA, Prandini MN, et al: Malignant transformation of pleomorphic xanthoastrocytoma: Case report. Arq Neuropsiquiatr 61:104-106, 2003
- Fouladi M, Jenkins J, Burger P, et al: Pleomorphic xanthoastrocytoma: Favorable outcome after complete surgical resection. Neuro-oncol 3:184-192, 2001
- Giannini C, Scheithauer BW, Burger PC, et al: Pleomorphic xanthoastrocytoma. What do we really know about it? Cancer 85:2033-2045, 1999
- Giannini C, Scheithauer BW, Lopes M, et al: Immunophenotype of pleomorphic xanthoastrocytoma. Am J Surg Pathol 26:479-485, 2002
- Glasser RS, Rojiani AM, Mickle JP, et al: Delayed occurrence of cerebellar pleomorphic xanthoastrocytoma after supratentorial pleomorphic xanthoastrocytoma removal. Case report. J Neurosurg 82:116-118, 1995
- Kepes JJ, Rubinstein LJ, Ansbacher L, et al: Histopathological features of recurrent pleomorphic xanthoastrocytomas: Further corroboration of the glial nature of this neoplasm. A study of 3 cases. Acta Neuropathol (Berl) 78:585-593, 1989
- Lee TT, Landy HJ, Bruce JH: Arteriovenous malformation associated with pleomorphic xanthoastrocytoma. Acta Neurochir (Wien) 138:590-591, 1996
- Levy RA, Allen R, McKeever P: Pleomorphic xanthoastrocytoma presenting with massive intracranial hemorrhage. AJNR Am J Neuroradiol 17:154-156, 1996
- Lipper MH, Eberhard DA, Phillips CD, et al: Pleomorphic xanthoastrocytoma, a distinctive astroglial tumor: Neuroradiologic and pathologic features. AJNR Am J Neuroradiol 14:1397-1404, 1993
- Nitta J, Tada T, Kyoshima K, et al: Atypical pleomorphic astrocytoma in the pineal gland: Case report. Neurosurgery 49:1458-1461, 2001
- Pahapill PA, Ramsay DA, Del Maestro RF: Pleomorphic xanthoastrocytoma: Case report and analysis of the literature concerning the efficacy of resection and the significance of necrosis. Neurosurgery 38:822-829, 1996
- Pierallini A, Bonamini M, Di Stefano D, et al: Pleomorphic xanthoastrocytoma with CT and MRI appearance of meningioma. Neuroradiology 41:30-34, 1999
- Prayson RA, Morris HH, III: Anaplastic pleomorphic xanthoastrocytoma. Arch Pathol Lab Med 122:1082-1086, 1998
- Sugita Y, Kepes JJ, Shigemori M, et al: Pleomorphic xanthoastrocytoma with desmoplastic reaction: Angiomatous variant. Report of two cases. Clin Neuropathol 9:271-278, 1990
- Whittle IR, Gordon A, Misra BK, et al: Pleomorphic xanthoastrocytoma. Report of four cases. J Neurosurg 70:463-468, 1989
- Zarate JO, Sampaolesi R: Pleomorphic xanthoastrocytoma of the retina. Am J Surg Pathol 23:79-81, 1999
