Racette Laboratory
Laboratory Focus
Our laboratory at Barrow Neurological Institute is within the Neuroepidemiology Research Program and focuses on epidemiologic studies that elucidate risk factors for neurodegenerative disease, with an emphasis on the environment and neurodegenerative disease. We employ a variety of methods in our research, including field-based epidemiology, advanced neuroimaging, and administrative data studies across three continents. Our highly multidisciplinary team aims to solve intractable problems, such as the etiology of neurodegenerative diseases using innovative research methods.
Active Research Projects
Epidemiology of Parkinsonism in Mn-Exposed Workers
Contributions to Science – Our team has developed the clinical research methodologies to reconstruct exposure history in Manganese (Mn)-exposed welders and has demonstrated that welders have a high prevalence of parkinsonian clinical signs. We have also shown that these clinical signs overlap with the clinical phenotype seen in idiopathic Parkinson’s disease patients, including reductions on Parkinson’s-related quality of life measures. Our team has demonstrated that inflammation, mediated by NOS2 gene expression, may underlie the pathophysiology of parkinsonism in Mn-exposed welders. These studies provide a clinical and pathophysiologic link between Mn neurotoxicity and idiopathic Parkinson’s disease.
Active Research Project – R01OH01166 (PI-Checkoway)
Manganese-Related Motor and Cognitive Toxicity Among Professional Welders
The purpose of this study is to investigate the association between Mn exposure and neurologic outcomes, as well as plasma proteins known to be associated with Parkinson disease.
Related Publications
- Hobson A, Seixas N, Sterling D, Racette BA. Estimation of particulate mass and manganese exposure levels among welders. Ann Occup Hyg. 2011 Jan;55(1):113-25. PubMed PMID: 20870928; PubMed Central PMCID: PMC3020674.
- Hobson AJ, Sterling DA, Emo B, Evanoff BA, Sterling CS, Good L, Seixas N, Checkoway H, Racette BA. Validity and Reliability of an Occupational Exposure Questionnaire for Parkinsonism in Welders. J Occup Environ Hyg 2009;6(6):324-331. PubMed PMID: 19288335; PubMed Central PMCID: PMC2879629.
- Harris RC, Lundin JI, Criswell SR, Hobson A, Swisher LM, Evanoff BA, Checkoway H, Racette BA. Effects of parkinsonism on health status in welding exposed workers. Parkinsonism Relat Disord. 2011 Nov;17(9):672-6. PubMed PMID: 21724446; PubMed Central PMCID: PMC3200492.
- Racette BA, Criswell SR, Lundin JI, Hobson A, Seixas N, Kotzbauer PT, Evanoff BA, Perlmutter JS, Zhang J, Sheppard L, Checkoway H. Increased risk of parkinsonism associated with welding exposure. Neurotoxicology. 2012 Oct;33(5):1356-61. PubMed PMID: 22975422; PubMed Central PMCID: PMC3651999.
- Racette BA, Aschner M, Guilarte TR, Dydak U, Criswell SR, Zheng W. Pathophysiology of manganese-associated neurotoxicity. Neurotoxicology. 2012 Aug;33(4):881-6. PubMed PMID: 22202748; PubMed Central PMCID: PMC3350837.
- Racette BA. Manganism in the 21st century: the Hanninen lecture. Neurotoxicology. 2014 Dec;45:201-7. PubMed PMID: 24148923; PubMed Central PMCID: PMC3992192.
- Lundin JI, Checkoway H, Criswell SR, Hobson A, Harris RC, Swisher LM, Evanoff BA, Racette BA. Screening for early detection of parkinsonism using a self-administered questionnaire: a cross-sectional epidemiologic study Neurotoxicology 2014;45:232-7. PubMed PMID: 24035927; PubMed Central PMCID: PMC3954448.
- Andruska KM, Racette AB. Neuromythology of Manganism. Curr Epidemiol Rep. 2015 Jun;2(2):143-148. PubMed PMID: 26046010; PubMed Central PMCID: PMC4450773.
- Searles Nielsen S, Checkoway H, Criswell SR, Farin FM, Stapleton PL, Sheppard L, Racette BA. Inducible nitric oxide synthase gene methylation and parkinsonism in manganese-exposed welders. Parkinsonism Relat Disord. 2015 Apr;21(4):355-60. PubMed PMID: 25634431; PubMed Central PMCID: PMC4512640.
- Baker MG, Criswell SR, Racette BA, Simpson CD, Sheppard L, Checkoway H, Seixas NS. Neurological outcomes associated with low-level manganese exposure in an inception cohort of asymptomatic welding trainees. Scand J Work Environ Health. 2015;41(1):94-101. PubMed PMID: 25380186; PubMed Central PMCID: PMC4354936.
Structural and Molecular Imaging of Mn Neurotoxicity
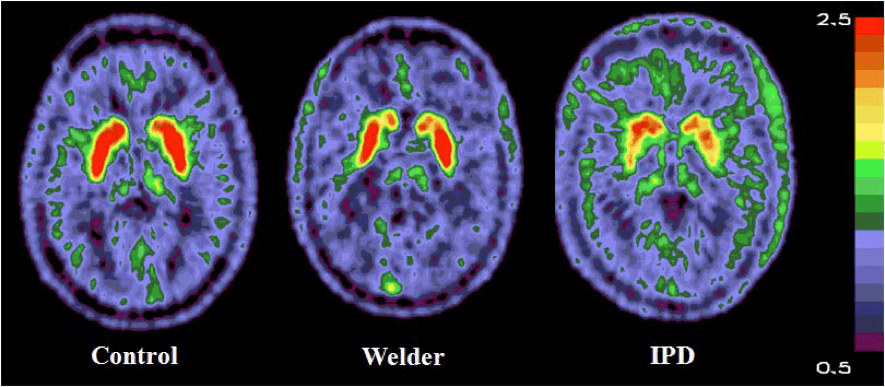
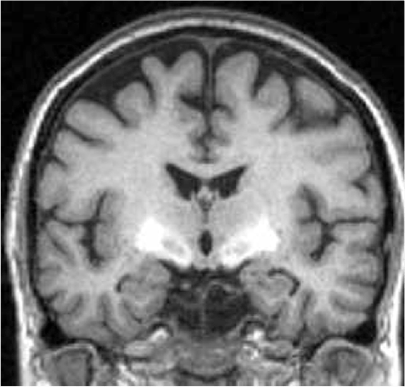
Contributions to Science – We have used brain MRI in Mn-exposed populations to demonstrate that basal ganglia signal intensity is associated with parkinsonism and Mn exposure, using in vivo and ex vivo T1 brain MRI. We have also found evidence of neuroinflammation in vivo on diffusion MRI. Using diffusion-weighted brain magnetic resonance imaging (MRI), we found that Mn-exposed welders have restricted diffusion in the globus pallidus and putamen as compared to non-welder reference participants.
We have used molecular imaging in Mn-exposed welders to understand the pathophysiology of Mn-associated neurotoxicity. We have found that Mn-exposed welders have lower caudate uptake of the radioligand 6-[18F]fluoro-L-dopa (FDOPA) than non-welder reference workers. We also investigated dopamine type 2 (D2) receptor binding in the brain in these workers using the radioligand [11C](N-methyl)benperidol (NMB). We found Mn dose-dependent upregulation of D2-receptors in the substantia nigra, and an association between clinical parkinsonism severity and D2-receptor binding in the substantia nigra. Similarly, we found inverse Mn dose-dependent binding of [11C]dihydrotetrabenazine (DTBZ) in the thalamus. These studies demonstrate that Mn exposure is associated with dopaminergic dysfunction and neurotoxic injury to the basal ganglia.
Current Projects
R01ES021488 (PI-Racette)
“Imaging Biomarkers of Neurotoxicity in Welders”
The purpose of years one through five was to investigate longitudinal changes FDOPA uptake in a cohort of manganese-exposed welders and to investigate cross-sectional DTBZ and NMB binding potentials in these same welders. In years six to 10, we are investigating the association between cumulative Mn exposure and change in DTBZ and NMB binding and whether PBR28 binding mediates those associations, in this same cohort.
R01ES030937 (PI-Tjalkens)
Encephalitic viral infection and susceptibility to dopaminergic neurotoxins [Virtual Consortium for Translational/Transdisciplinary Environmental Research (ViCTER)]
The purpose of this study is to investigate the association between occupational Mn exposure and neuroinflammation using PBR28 PET and the role of neurotrophic viruses as mediator of Mn-induced neuroinflammation.
R01ES029524 (PI- Criswell)
Novel PET Markers of Cognitive Impairment in Manganese Neurotoxicity
This proposal employs state-of-the-art molecular imaging methods combined with an innovative statistical approach to understand the dual roles of the cholinergic and dopaminergic systems in Mn-induced cognitive impairment.
Related Publications
- Criswell SR, Perlmutter JS, Videen TO, Moerlein SM, Flores HP, Birke AM, Racette BA. Reduced Uptake of [18F]FDOPA PET in Asymptomatic Welders with Occupational Manganese Exposure. Neurology 2011; 6(15):1296-301.
- Criswell SR, Perlmutter JS, Crippin JS,Videen TO, Moerlein SM, Flores HP, Birke AM, Racette BA. Reduced Uptake of [18F]FDOPA PET in End Stage Liver Disease with Elevated Manganese Levels. Arch Neurol 2012;69(3):394-7.
- Criswell SR, Nelson G, Gonzalez-Cuyar LF, Huang J, Shimony JS, Checkoway H, Simpson CD, Dills R, Seixas NS, Racette BA.Ex vivo magnetic resonance imaging in South African manganese mine workers. Neurotoxicology 2015; 49:8-14.
- Criswell SR, Nielsen SS, Warden M, Perlmutter JS, Moerlein SM, Flores HP, Huang J, Sheppard L, Seixas N, Checkoway H, Racette BA. [18F]FDOPA Positron Emission Tomography in Manganese-Exposed Workers. Neurotoxicology 2018:64:43-49.
- Criswell SR, Warden MN, Searles Nielsen S, Perlmutter JS, Moerlein SM, Sheppard L, Lenox-Krug J, Checkoway H, Racette BA. Selective D2 Receptor PET in Manganese-Exposed Workers. Neurology; 2018;91:e1022-e1030.
- Criswell SR, Nielsen SS, Warden MN, Perlmutter JS, Moerlein SM, Sheppard L, Lenox-Krug J, Checkoway H, Racette BA. [11C]dihydrotetrabenazine Positron Emission Tomography in Manganese-Exposed Workers. JOEM, 2020; 62(10):788-794.
- Criswell SR, Searles Nielsen S, Dlamini WW, Warden MN, Perlmutter JS, Sheppard L, Moerlein SM, Lenox-Krug J, Checkoway H, Racette BA. Principal Component Analysis of Striatal and Extrastriatal D2 Dopamine Receptor Positron Emission Tomography in Manganese Exposed Workers. Toxicol Sci 2021; 182(1):132-141.
Over 15 years ago, while at the London School of Tropical Medicine and Hygiene, Dr. Brad Racette began to collaborate with Professor Gill Nelson, now at the University of the Witwatersrand in Johannesburg, South Africa. Their collaboration initially focused on creating a brain bank of Mn miners but has expanded to include the impact of environmental Mn on neurologic health in adults and children. Recently, they began new partnerships with investigators in the Agincourt cohort in rural northern South Africa.
Neuropathology of Chronic Mn Exposure
Contributions to Science – In a collaboration with the University of the Witwatersrand in Johannesburg, South Africa, we have developed a neuropathology autopsy research program in South African Mn mineworkers and have collected over 80 brains from deceased miners. Our initial results suggest that Mn mineworkers have lower neuron and astrocyte density and higher microglial density in the striatum. This suggests that Mn may induce a proinflammatory balance in the corpus striatum that ultimately leads to neuronal injury. Although these workers have typical high signal in their basal ganglia on MRI, the Mn concentration is the same in Mn miners as compared to non-Mn miners. These workers demonstrate a similar clinical phenotype to our Mn-exposed welders, associated reductions in quality of life, and a U-shaped dose-response relation between parkinsonism and exposure, consistent with the healthy worker effect.
Related Publications
- Nelson G, Criswell SR, Zhang J, Murray J, Racette BA. Research capacity development in South African manganese mines to bridge exposure and neuropathologic outcomes. Neurotoxicology. 2012 Aug;33(4):683-6. PubMed PMID: 22313906; PubMed Central PMCID: PMC3411927.
- Gonzalez-Cuyar LF, Nelson G, Criswell SR, Ho P, Lonzanida JA, Checkoway H, Seixas N, Gelman BB, Evanoff BA, Murray J, Zhang J, Racette BA. Quantitative neuropathology associated with chronic manganese exposure in South African mine workers. Neurotoxicology. 2014 Dec;45:260-6. PubMed PMID: 24374477; PubMed Central PMCID: PMC4072755.
- Criswell SR, Nelson G, Gonzalez-Cuyar LF, Huang J, Shimony JS, Checkoway H, Simpson CD, Dills R, Seixas NS, Racette BA. Ex vivo magnetic resonance imaging in South African manganese mine workers. Neurotoxicology. 2015 Jul;49:8-14. PubMed PMID: 25912463; PubMed Central PMCID: PMC4523412
- Dlamini WW, Nelson G, Nielsen SS, Racette BA. Manganese exposure, parkinsonian signs, and quality of life in South African mine workers. AJIM 2020; 63(1):36-43.

Current Projects
R01ES026891 (PI-Racette)
Manganese-Induced Neurotoxic Effects Research in South Africa (MINERS)
The purpose of this study is to perform neuropathologic stereology studies in deceased Mn miner and non-Mn miner brains to investigate the relationship between astrocyte, neuron, and microglial cell counts and Mn exposure. We will also use quantitative immunofluorescence to investigate markers of neuronal health and markers of neurotoxicity in these neuropathologic specimens and will measure tissue metal content to investigate the association with quantitative ex vivo MRI metal sensitive sequences.
Neurologic Health Effects of Environmental Mn Exposures
Contributions to Science – Our team has recently published several high-impact studies from our SMELTER cohort, in which we developed and validated the tools used to investigate the health effects of environmental Mn in South Africa. We followed these manuscripts with publications, demonstrating an association between environmental Mn exposure and parkinsonian motor outcomes, depression, and cognitive control dysfunction.
Related Publications
- Nelson G, Ndlovu N, Christofides N, Hlungwani TM, Faust I, Racette BA. Validation of Parkinson’s Disease-related Questionnaires in South Africa. Parkinsons Dis 2020;2020:7542138. PubMed PMID: 32617145; PubMed Central PMCID: PMC7306845
- Dlamini WW, Searles Nielsen S, Ushe M, Nelson G, Racette BA. A Rapid Motor Task-Based Screening Tool for Parkinsonism in Community-Based Studies. Front Neurol 2021; May 13;12:653066. doi: 10.3389/fneur.2021.653066. eCollection 2021.
- Racette BA, Nelson G, Dlamini WW, Prathibha P, Turner J, Ushe M, Checkoway H, Sheppard L, Nielsen SS. Severity of Parkinsonism Associated with Environmental Manganese Exposure. Environ Health 2021;20:27. PubMed PMID: 33722243; PubMed Central PMCID: PMC7962371
- Racette BA, Nelson G, Dlamini WW, Hershey T, Prathibha P, Turner JR, Checkoway H, Sheppard L, Searles Nielsen S. Depression and anxiety in a manganese-exposed community. Neurotoxicology 2021;85:222-223. PubMed PMID: 34087333; PubMed Central PMCID: PMC8635218
- Racette BA, Nelson G, Dlamini WW, Hershey T, Prathibha P, Turner JR, Checkoway H, Sheppard L, Searles Nielsen S. Environmental manganese exposure and cognitive control in a South African population. Neurotoxicology 2022;;89:31-40. PubMed PMID: 34999155;
Current Projects
R01ES025991 (PI-Racette)
“Motor and Cognitive Health Outcomes in a Mn-Exposed African Community”
The purpose of this study is to investigate the motor, cognitive, and mood health effects associated with environmental manganese (Mn) exposure from a Mn smelter in a mixed-race South African community.
“The effect of exposure to environmental manganese on the neurobehavioral function of children in Meyerton, South Africa”
The purpose of this study is to investigate motor and cognitive health outcomes in the Meyerton cohort in relation to cumulative environmental Mn exposure.
Social Determinants of Health and Parkinsonism
We are collaborating with investigators from the Agincourt cohort in rural northern South Africa to investigate the epidemiology of parkinsonism in a population-based rural African population. These studies focus on the impact of life stressors and social determinants of health on age-related parkinsonism.
Current Project
Africa Initiative Pilot Grant Program (PI-Racette)
Social Determinants of Parkinsonism in Rural South Africa
The purpose of this pilot grant is to develop preliminary data on the relation between social determinants of health and clinical parkinsonism in the “Health and Aging in Africa: Longitudinal Studies of an INDEPTH Community” cohort, nested within the Agincourt cohort, in the Mpumalanga Province in rural northern South Africa.
Our laboratory has pioneered the use of Medicare administrative data to investigate Parkinson’s and other neurodegenerative diseases. We use case control or retrospective cohort designs to conduct association studies, predictive modelling, and geospatial epidemiology.
Prodromal PD
Our team has recently published several important studies using Medicare data from the five-year prodromal PD disease period. We developed a predictive model using only medical claims data that demonstrated high sensitivity and specificity for PD. In addition, we have used this prodromal PD dataset to demonstrate the importance of health care utilization as a confounder in PD association studies. We have also used Medicare Part D pharmacy claims data to investigate risk of PD in relation to prescription medications. These latter studies are influencing the direction of neuroprotective clinical trials in the U.S.
Related Publications
- Searles Nielsen S, Warden MN, Camacho-Soto A, Willis AW, Wright BA, Racette BA. A predictive model to identify Parkinson disease from administrative claims data. Neurology. 2017;89(14):1448-1456. PubMed PMID: 28864676; PubMed Central PMCID: PMC5631173
- Camacho-Soto A, Warden MN, Searles Nielsen S, Salter A, Brody DL, Prather H, Racette BA. Traumatic brain injury in the prodromal period of Parkinson’s Disease: A large epidemiological study using Medicare data. Ann Neurol. 2017;82(5):744-754. PubMed PMID: 29024046; PubMed Central PMCID: PMC5812286
- Camacho-Soto A, Gross A, Searles Nielsen S, Miller AN, Warden MN, Salter A, Racette BA. Fractures in the prodromal period of Parkinson disease. Neurology. 2020;94(23):e2448-e2456. PubMed PMID: 32345729; PubMed Central PMCID: PMC7455361
- Gross A, Racette BA, Camacho-Soto A, Dube U, Searles Nielsen S. Use of medical care biases associations between Parkinson disease and other medical conditions. Neurology. 2018;90(24):e2155-e2165. PubMed PMID: 29743207; PubMed Central PMCID: PMC5996836
Current Projects
PD190057, DoD (PI-Racette)
Parkinson’s Risk Estimation Using Digital Diagnosis Codes and Treatments
The purpose of this study is to develop a predictive model to identify PD patients during their prodromal disease period, using ICD10 and CPT codes obtained from the Center for Medicare and Medicaid Services. A second aim of this project is to identify medications associated with a lower risk of PD.
Environmental Air Pollution and PD Risk
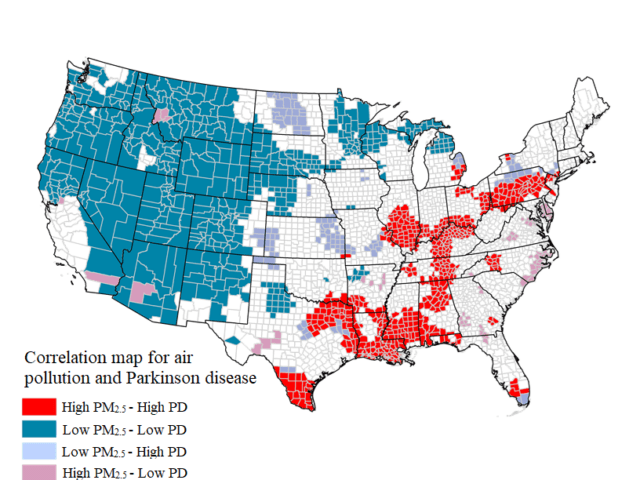
Using Medicare to identify all patients with PD over age 65 in the U.S., we demonstrated a highly significant geographic clustering of prevalent and incident Parkinson’s disease in the Midwestern and Eastern U.S. Using EPA-reported industrial emissions data, we also demonstrated that those living in counties with high industrial Mn emissions had a higher incidence of Parkinson’s disease than those living in counties with low Mn emissions, after adjusting for covariates. We also found that survival in Parkinson’s disease patients living in counties with high Mn emissions was lower than those living in counties with low Mn emissions.
These studies suggest that environmental Mn exposure is associated with a higher risk of Parkinson’s disease and reduced survival, possibly due to a more treatment-resistant clinical phenotype. Our current work focuses on risk of PD in relation to PM2.5 and variety of novel environmental exposures.

Related Publications
- Wright Willis A, Evanoff BA, Lian M, Criswell SR, Racette BA. Geographic and ethnic variation in Parkinson disease: a population-based study of U.S. Medicare beneficiaries. Neuroepidemiology. 2010;34(3):143-51. PubMed PMID: 20090375; PubMed Central PMCID: PMC2865395.
- Willis AW, Evanoff BA, Lian M, Galarza A, Wegrzyn A, Schootman M, Racette BA. Metal emissions and urban incident Parkinson disease: a community health study of Medicare beneficiaries by using geographic information systems. Am J Epidemiol. 2010 Dec 15;172(12):1357-63. PubMed PMID: 20959505; PubMed Central PMCID: PMC2998201.
- Willis AW, Schootman M, Kung N, Evanoff BA, Perlmutter JS, Racette BA. Predictors of survival in patients with Parkinson disease. Arch Neurol. 2012 May;69(5):601-7. PubMed PMID: 22213411; PubMed Central PMCID: PMC3599783.
Current Projects
MJFF-000939 (PI-Nielsen)
A Nationwide Geographic Cluster Analysis of Incident Parkinson’s Disease (MAP-PD)
In this nationwide study based on administrative claims data from a population-based sample of Medicare beneficiaries, we will investigate environmental risk factors for Parkinson’s disease through a multipronged approach. We will conduct a comprehensive series of geographic cluster analyses, followed by formal hypothesis testing of the associations between the most promising candidate environmental exposures and Parkinson’s disease risk.
Health Services Utilization in Patients with Parkinson’s Disease
We have published several high-impact studies investigating the use of neurologic specialty services in patients with Parkinson’s disease using Medicare data. In these studies, we found that more than 50% of patients with Parkinson’s disease do not see a neurologist and that neurologist care is associated with improved survival and reduced risk of PD related comorbidities.
We have also demonstrated that minorities, women, and those from low socioeconomic neighborhoods access specialty care less frequently and are less likely to be treated with advanced surgical therapies.
Related Publications
- Willis AW, Schootman M, Evanoff BA, Perlmutter JS, Racette BA. Neurologist care in Parkinson disease: a utilization, outcomes, and survival study. Neurology. 2011 Aug 30;77(9):851-7. PubMed PMID: 21832214; PubMed Central PMCID: PMC3162639.
- Willis AW, Schootman M, Tran R, Kung N, Evanoff BA, Perlmutter JS, Racette BA. Neurologist-associated reduction in PD-related hospitalizations and health care expenditures. Neurology. 2012 Oct 23;79(17):1774-80. PubMed PMID: 23054239; PubMed Central PMCID: PMC3475618.
- Willis AW, Schootman M, Kung N, Evanoff BA, Perlmutter JS, Racette BA. Predictors of Survival in Parkinson Disease. Arch Neurol 2012; (5):601-7.
- Willis AW, Schootman M, Kung N, Wang XY, Perlmutter JS, Racette BA. Disparities in deep brain stimulation surgery among insured elders with Parkinson disease. Neurology. 2014 Jan 14;82(2):163-71. PubMed PMID: 24336138; PubMed Central PMCID: PMC3897433.
- Harris-Hayes M, Willis AW, Klein SE, Czuppon S, Crowner B, Racette BA. Relative mortality in U.S. Medicare beneficiaries with Parkinson disease and hip and pelvic fractures. J Bone Joint Surg Am. 2014 Feb 19;96(4):e27. PubMed PMID: 24553896; PubMed Central PMCID: PMC3918936.
- Safarpour D, Dylan P, Thibault DP, DeSanto CL, Boyd CM, Ray Dorsey ER, Racette BA, Willis AW. Nursing Home and End-of-Life Care in Parkinson Disease. Neurology 2015; 85(5):413-419. PubMed PMID: 26138947; PubMed Central PMCID: PMC4534080.
- Morris M, Willis AW, Nielsen SS, McCann F, Birke, A, Racette BA. Physician response to a medication alert system in inpatients with levodopa treated diseases. Neurology 2015; 85(5):420-424.
Current Projects
MJFF-020718 (PI- Camacho-Soto)
“From the population to the community of providers: Identifying barriers to care and social determinants of health in neurological ‘deserts’ across the United States”
Using a nationwide population-based dataset, we will investigate how social determinants of health (SDOH) affect fracture risk, a well-described comorbidity in persons with Parkinson’s disease (PD). We will use these data to inform provider and persons with PD surveys to identify barriers to care.
We are using multiple methods and study designs to investigate risk factors for amyotrophic lateral sclerosis (ALS) and to identify potential disease-modifying medications in Medicare data. Our recent work demonstrates that the incidence of ALS may be higher than previously thought. Notably, fewer than 40% of cases ever had an ALS diagnosis from a neurologist and 40% survived less than one year after diagnosis, with 25.5% of these cases surviving no more than six months. The oldest and most economically marginalized patients were the least likely to receive care from a neurologist. Our current work uses a “bench-to-beside and back” approach to identify FDA-approved medications that could be repurposed as disease-modifying therapies. Other studies investigate environmental risk factors for ALS, using cutting-edge geospatial techniques.
Related Publications
- Camacho-Soto A, Searles Nielsen S, Faust IM, Bucelli RC, Miller TM, Racette BA, Incidence of Amyotrophic Lateral Sclerosis in Medicare-Age Adults. Muscle Nerve. 2022;66(3):289-296.
Previous Funding
Northeast ALS Consortium (PI-Racette)
“Tambourine”
The purpose of this pilot grant is to investigate racial and geographic risk factors for ALS using a population-based Medicare dataset.
Hope Center for Neurologic Disorders (PI-Racette)
Identification of Repurposed Prescription Medications as Neuroprotective Therapy for Amyotrophic Lateral Sclerosis
The goal of this project is to identify prescription medications in Medicare Part D associated with a lower risk of amyotrophic lateral sclerosis. The top candidate medications will be tested in a mouse model for disease modification.
In these studies, we are expanding our Medicare data studies to investigate risk factors for Alzheimer’s disease (AD) and to attempt to identify medications from the Medicare formulary that could be repurposed as disease-modifying therapies for patients with AD. We are particularly interested in understanding environmental risk factors for AD and health care utilization in this devastating disease.
Current Projects
Cure Alzheimer’s Fund (PI-Racette)
Harnessing Big Data to Understand Alzheimer’s Disease Risk
The purpose of this study is to investigate the risk of Alzheimer’s disease in relation to meningitis and medications in the Medicare formulary medical claims data obtained from the Center for Medicare and Medicaid Services.
Dr. Racette has published extensively on the genetics of Parkinson’s disease, including numerous large family genetic studies and genetic association studies.
Related Publications
- Ahmed I, Lee PC, Lill CM, Searles Nielsen S, Artaud F, Gallagher LG, Loriot MA, Mulot C, Nacfer M, Liu T, Biernacka JM, Armasu S, Anderson K, Farin FM, Lassen CF, Hansen J, Olsen JH, Bertram L, Maraganore DM, Checkoway H, Ritz B, Elbaz A. Lack of replication of the GRIN2A-by-coffee interaction in Parkinson disease. PLoS Genet. 2014 Nov;10(11):e1004788. PubMed PMID: 25412286; PubMed Central PMCID: PMC4238979.
- Parsian A, Racette B, Goldsmith LJ, Perlmutter JS. Parkinson’s disease and apolipoprotein E: Possible association with dementia but not age of onset. Genomics. 2002;79(3):458_61. PubMed PMID: 11863377.
- Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a Screening Questionnaire for Genetic Linkage Studies of Parkinson’s Disease. American Journal of Medical Genetics (Neuropsychiatric section) 1999;88:539-543. PubMed PMID: 10490713.
- Racette BA, Rundle M, Wang JC, Goate A, Saccone NL, Farrer M, Lincoln S, Hussey J, Lin J, Suarez B, Parsian A, Perlmutter JS. A Multi-incident, Old-Order Amish family with PD. Neurology 2002; 58: 568-574. PubMed PMID: 11865134.
- Parsian A, Racette B, Zhang ZH, Rundle MR, Perlmutter JS. Association of variations in monoamine oxidases A and B with Parkinson’s disease subgroups. Genomics 2004; 83(3):454-60. PubMed PMID: 14962671.
- Sinha R, Racette B, Perlmutter JS, Parsian A. Prevalence of parkin gene mutations and variations in IPD. Parkinsonism Relat Disord 2005;11(6):341-347. PubMed PMID: 16019250.
- Karamohamed S, Latourelle JC, Racette BA, Perlmutter JS, Wooten GF, Lew MF, Klein C, Shill H, Golbe LI, Mark MH, Guttman M, Nicholson G, Wilk JB, Saint-Hilaire MH, DeStefano AL, Prakash R, Williamson S, Tobin J, Suchowersky O, Labelle N, Growdon JH, Singer C, Watts RL, Goldwurm S, Pezzoli G, Baker KB, Giroux ML, Pramstaller PP, Burn DJ, Chinnery PF, Sherman S, Vieregge P, Litvan I, Gusella JF, Myers RH, Parsian A. BDNF genetic variants are associated with onset-age of familial Parkinson’s disease: GenePD study. Neurology 2005;65(11):1823-1825. PubMed PMID: 16344533.
- Racette BA, Good LM, Antenor J, McGee-Minnich L, Moerlein SM, Videen TO, Perlmutter JS. [18F]FDOPA PET as an Endophenotype for Parkinson’s Disease Linkage Studies. American Journal of Medical Genetics (Neuropsychiatric section) 2006; 141(3):245-9. PubMed PMID: 16528749; PubMed Central PMCID: PMC2646004.
- Racette BA, Good LM, Kissel AM, Criswell SR, Perlmutter JS. A Population Based Study of Parkinsonism in an Amish Community. Neuroepidemiology 2009; 33(3):225-230. PubMed PMID: 19641327; PubMed Central PMCID: PMC2826445.

Contact Information
Brad Racette, MD, FAAN
Kemper and Ethel Marley Professor and Chair of Neurology
350 West Thomas Road
Phoenix, Arizona 85013
Email: NeuroepidemiologyRequests@BarrowNeuro.org