
Stokes Laboratory
Laboratory Focus
Imaging-based biomarkers play a critical role in many neuropathologies, ideally giving insight into the structural and functional changes in the brain that lead to clinical manifestations of disease. Conventional magnetic resonance imaging (MRI) is sensitive to downstream indicators of neuropathology but often lacks specificity to the underlying causes of disease. More advanced imaging methods provide unique and complementary information that is directly related to pathophysiology and could have a significant clinical impact that crosscuts all aspects of patient management.
Our research focuses on developing, validating, and translating advanced MRI acquisition and analysis methods to noninvasively assess neurological diseases and disorders, including Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis. These advanced imaging methods include methods to interrogate brain hemodynamics (perfusion MRI), microstructure (diffusion MRI), and brain function (functional MRI). The long-term goal of our research is to develop advanced imaging biomarkers that can inform on the underlying disease pathophysiology.
Advanced Imaging Biomarkers in Alzheimer’s Disease
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder and is the most common cause of dementia. AD symptoms generally begin with memory difficulties and evolve to include multiple domains of cognition, eventually affecting activities of daily living. Clinical outcomes in AD remain exceedingly poor, in part due to limited understanding of its neuropathological underpinnings.
Patients may also experience mild cognitive impairment (MCI), a related syndrome characterized by objective memory decline with relative preservation of other cognitive domains and functional activities. MCI is associated with an increased risk of conversion to AD, and the MCI phase represents an opportunity for potential early intervention.
Imaging-based biomarkers using MRI play a critical role in assessing Alzheimer’s-related pathological changes, but current biomarkers are limited in their diagnostic and prognostic ability, particularly in early disease stages when intervention would be most beneficial. Work in our lab aims to overcome these challenges by developing novel MRI biomarkers with high biological and pathological specificity to better understand both aging and AD pathology. These biomarkers relate to microstructural integrity, brain network connectivity, and cerebrovascular health.
Microstructural Changes in Aging and AD
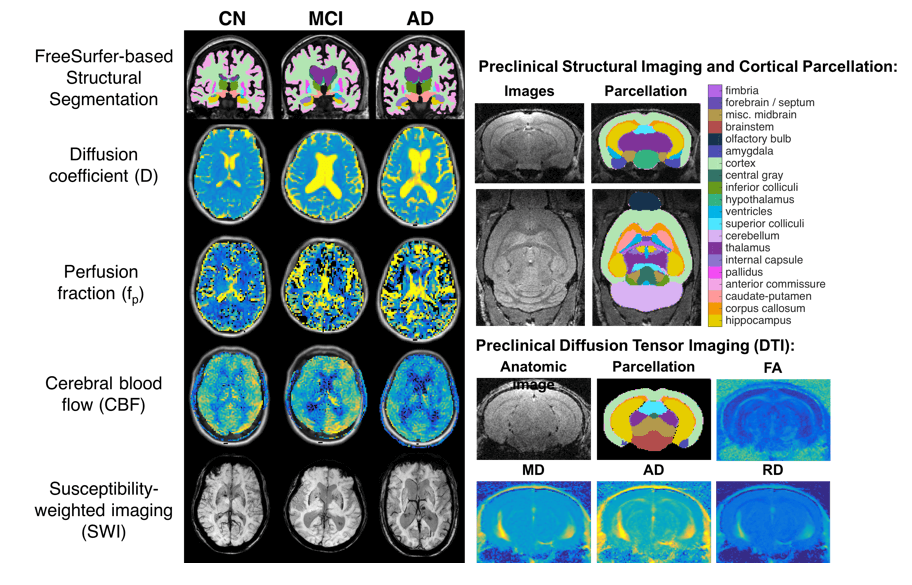
Diffusion MRI (dMRI) comprises a set of complementary non-invasive techniques that inform on microstructural characteristics via diffusivity of water in the brain. Studies have shown that changes in dMRI-related metrics precede both neuronal loss and symptom onset in MCI and AD, suggesting their potential role as early biomarkers. Unfortunately, Alzheimer’s disease leads to complex pathological changes that are not fully captured by current dMRI models, necessitating the development of more advanced models that account for various aspects of Alzheimer’s disease pathology.
In separate studies, we have shown that adding free-water and perfusion components to dMRI models accounts for structural (neurodegeneration) and functional (perfusion) changes associated with Alzheimer’s disease and thus improves the sensitivity and specificity of the resulting biomarkers.
For example, we recently showed that free water corrected dMRI biomarkers are more accurate and better reflect the underlying pathology of AD. Ongoing work in our lab focuses on developing and validating more advanced dMRI models for use in MCI and AD.
Functional connectivity changes in aging and AD
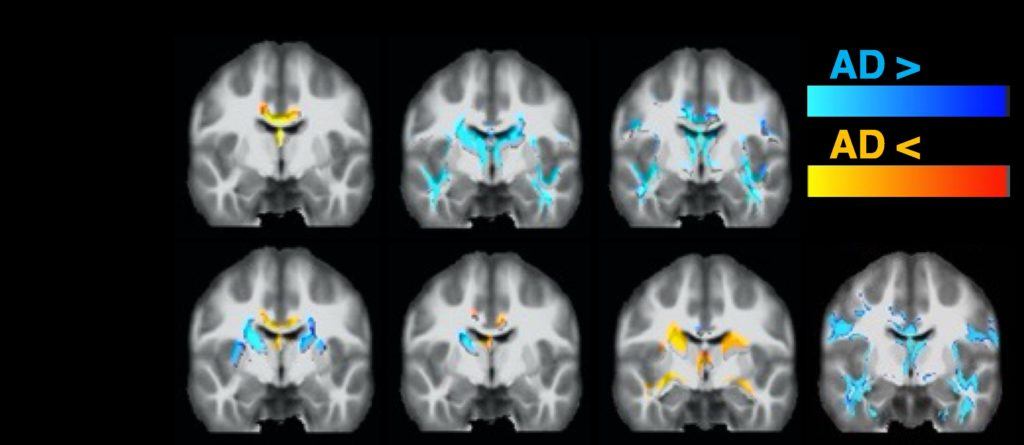
Functional MRI (fMRI) is widely used to map synchronous fluctuations in brain activity, revealing brain connectivity in the resting state or response to functional activation during tasks. In the absence of a task, functional brain networks can be identified by analysis of synchronous fluctuations in the BOLD signal, which is particularly advantageous in cognitively impaired populations.
Numerous studies of resting-state functional connectivity have shown altered networks in MCI and AD, particularly in the default mode network, salience network, and frontoparietal network, as well as associated with the medial temporal lobe. One of the drawbacks of existing methods is signal dropouts in critical regions of interest, including in the medial temporal lobe.
Work in our lab is focused on improving fMRI metrics using advanced methods to recover signal in those critical regions. Relatedly, we are interested in combining both neuropsychological measures and neuroimaging (MRI) biomarkers to improve sensitivity and specificity to early pathological changes. For example, recent studies have suggested that finger tapping abnormalities may have diagnostic value for separating patients with subjective memory complaints from those with AD and objective memory impairment.
Combining neuropsychological metrics with an advanced MRI protocol may provide insight into the underlying early neuropathological changes associated with these motor changes.
Cerebrovascular Changes in Aging and AD
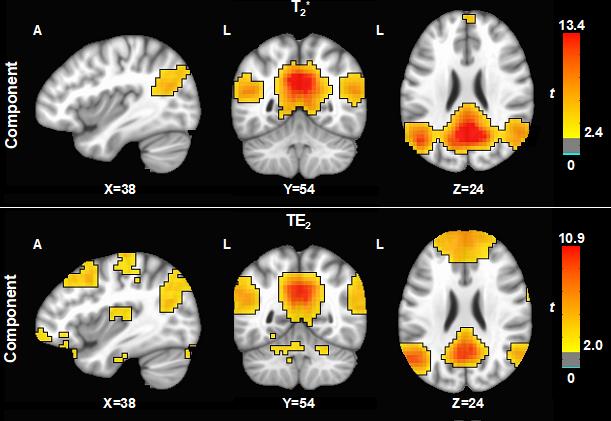
Cerebrovascular dysfunction is increasingly recognized as a contributing factor in the development of AD pathology, with deleterious downstream effects on the neurovasculature, amyloid and tau pathology, and neuroinflammation. Vascular changes are of particular interest as they are thought to occur early in the pathological cascade, prior to cognitive decline, and could potentially be managed therapeutically.
Research in our lab is focused on two complementary aspects of cerebrovascular health: brain perfusion and cerebrovascular reactivity (CVR). In particular, we are interested in developing new MRI methods that are sensitive to both global and microvascular changes in perfusion and CVR. The goal of this approach is to measure perfusion and CVR changes in healthy aging and MCI cohorts on both a global and microvascular scale.
Advanced Imaging Biomarkers in Parkinson’s Disease
Parkinson’s disease (PD) is a progressive neurodegenerative disorder, and patients with PD experience significant motor and non-motor symptoms. Treatment for PD has historically focused on ameliorating the classic motor symptoms; however, patients also experience a wide range of non-motor symptoms, some of which are found to be more debilitating than the motor symptoms themselves, severely burdening patients and their families.
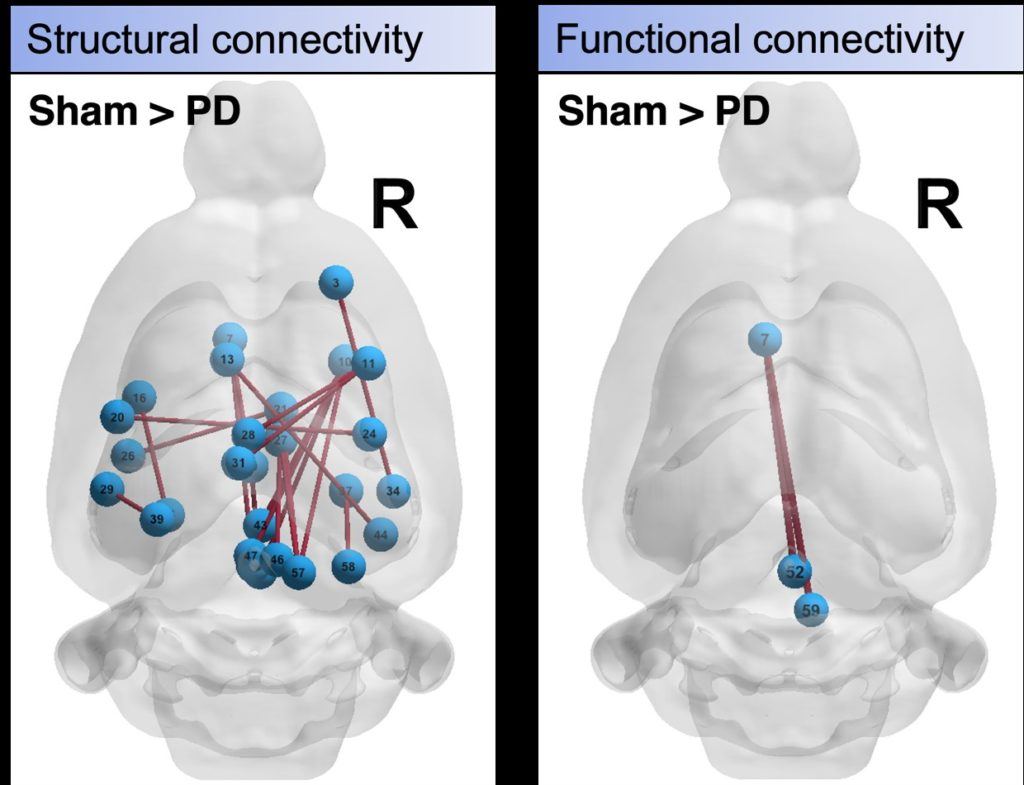
Broadly speaking, these symptoms can be traced to the loss of nigral dopamine neurons, but it is increasingly recognized that PD involves widespread circuit dysfunction that extends beyond the dying nigrostriatal tract.
Imaging-based biomarkers play a critical role in assessing Parkinson’s-related pathological changes, but current biomarkers are limited in their diagnostic and prognostic ability, particularly in early disease stages when intervention would be most beneficial. Functional magnetic resonance imaging (fMRI) enables the study of brain activation and has been widely used to study global functional network changes in Parkinson’s disease. However, standard fMRI is limited in its ability to robustly measure subtle changes with disease, in part due to low sensitivity and specificity; furthermore, interpretation of standard fMRI is challenging due to the indirect link between neuronal function and MRI signal change.
This lack of robust direct biomarkers is a critical gap that ultimately limits our ability to understand the underlying pathological changes, as well as evaluate emerging therapies. To overcome these limitations, our lab has developed and optimized an advanced multi-contrast fMRI method that provides high contrast sensitivity, as well as distinct microvascular sensitivity. By coupling this method with pharmacological and chemogenetic manipulations, a direct link between fMRI-based functional networks and underlying neuronal function can be inferred.
These studies provide critical insight into functional network changes that occur over different vascular scales and via different neurotransmitter populations, which are implicated in the multifaceted nature of Parkinson’s disease that contributes to both motor and non-motor symptoms. As functional brain network dysfunction is widely observed in Parkinson’s disease, this integrative approach will enable the development of robust biomarkers of Parkinson’s disease with well-characterized pathophysiological origins, which is a critical shortcoming of current technologies.
Advanced Imaging Biomarkers in Multiple Sclerosis
Multiple sclerosis (MS) is a chronic, debilitating disease of the central nervous system. Patients with MS experience a wide range of clinical and pathological symptoms over an unpredictable time-course often spanning decades. Relapsing-remitting MS is characterized by periods of new or worsening neurologic symptoms with a relative return to baseline between episodes.
Pathologically, MS is characterized by neuroinflammation, focal demyelination, gliosis, axonal degeneration, and neuronal loss. Remyelination of MS lesions is associated with improvement of symptoms but is highly variable; thus, therapies that foster remyelination represent an opportunity for repair before irreversible damage and decline.
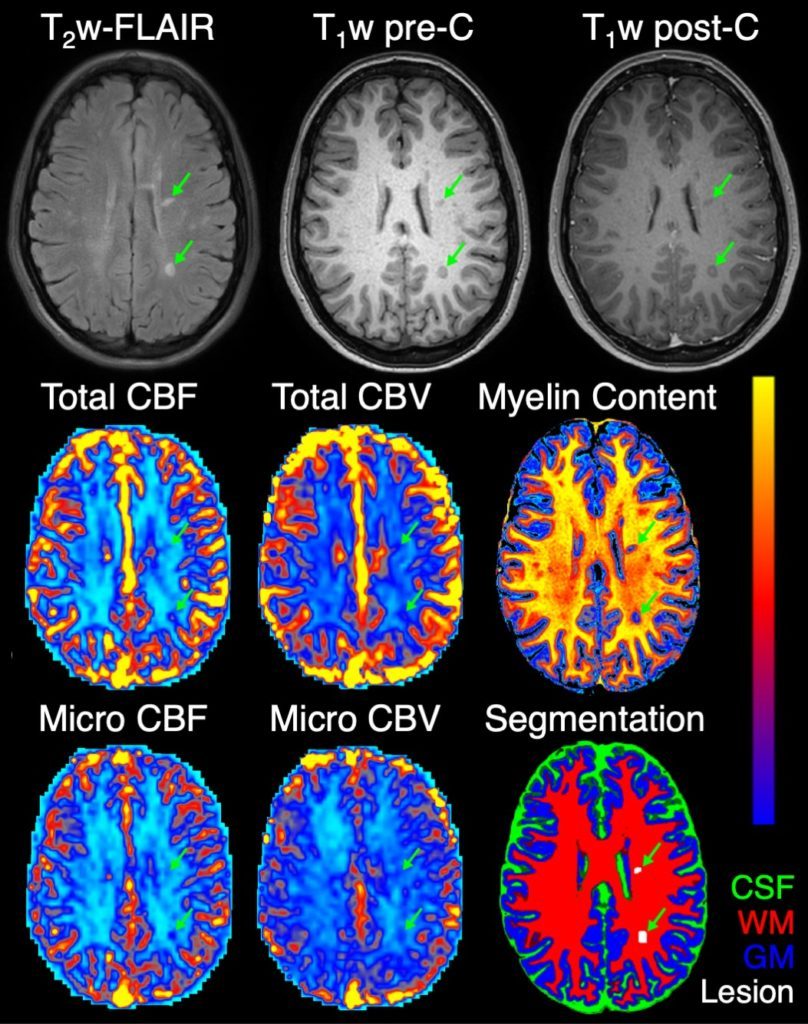
Neuroimaging plays a critical role in the clinical management of MS, but conventional MRI lacks pathological specificity to the factors that exacerbate demyelination or promote remyelination. The development of MS-specific biomarkers remains a highly relevant target and may impact patient diagnosis, prognosis, disease management, therapeutic planning, and clinical trial outcomes.
Given the importance of myelination, MRI biomarkers of myelin integrity have been developed for use in clinical trials. Unfortunately, these biomarkers reflect static levels of myelin and cannot predict demyelination or remyelination processes. Recent studies have suggested that remyelination relies on adequate tissue perfusion. While altered perfusion has been reported in MS, the relationship between perfusion and myelin has not been fully characterized in vivo.
To robustly assess cerebral perfusion across the brain, our lab has developed and validated an advanced MRI method to measure perfusion across varying vascular scales, including at the microvascular level. Using this method, we recently showed that microvascular perfusion in MS lesions is associated with reduced microstructural integrity, a key feature of demyelination. Ongoing work in our lab – in collaboration with the Dortch Lab – focuses on investigating the role of perfusion in demyelination and remyelination using advanced MRI biomarkers.

Contact Information
Ashley Stokes, PhD
Assistant Professor, Neuroimaging Research
Barrow Neurological Institute
350 West Thomas Road
Phoenix, Arizona 85013
Ashley.Stokes@DignityHealth.org
We are currently recruiting both graduate students and postdoctoral fellows. Our research focuses on the development, validation, and translation of advanced MRI methods in Parkinson’s disease, multiple sclerosis, and Alzheimer’s disease. These advanced methods involve acquisition and analysis of perfusion, diffusion, and functional MRI data in both preclinical disease models and clinical applications. Graduate students and fellows will work in a highly collaborative environment, with imaging-focused scientists in the Barrow Neuroimaging Innovation Center and with neuroscientists and neurologists at Barrow.
We are also accepting applications from motivated individuals who can commit to volunteering in the laboratory, but these spaces are limited. Volunteers in the Stokes Laboratory can expect to participate in a variety of research-related activities, including contribution to a research project.
The ISMRM Open Science Initiative for Perfusion Imaging (OSIPI): Results from the OSIPI-Dynamic Contrast-Enhanced challenge
Date: 12/2023
Authors: Eve S. Shalom, Harrison Kim, Rianne A. van der Heijden, Zaki Ahmed, Reyna Patel, David A. Hormuth, Julie C. DiCarlo, Thomas E. Yankeelov, Nicholas J. Sisco, Richard D. Dortch, Ashley M. Stokes, Marianna Inglese, Matthew Grech-Sollars, Nicola Toschi, Prativa Sahoo, Anup Singh, Sanjay K. Verma, Divya K. Rathore, Anum S. Kazerouni, Savannah C. Partridge, Eve LoCastro, Ramesh Paudyal, Ivan A. Wolansky, Amita Shukla-Dave, Pepijn Schouten, Oliver J. Gurney-Champion, Radovan Jiřík, Ondřej Macíček, Michal Bartoš, Jiří Vitouš, Ayesha Bharadwaj Das
Assessment of complementary white matter microstructural changes and grey matter atrophy in a preclinical model of Alzheimer’s disease
Date: 09/2023
Authors: Maurizio Bergamino, Megan R. Nelson, Asfia Numani, Matthew Scarpelli, Deborah Healey, Debbie R. Healey, Alberto Fuentes, Gregory Turner, Ashley M. Stokes
White Matter Microstructural Differences between Essential Tremor and Parkinson Disease, Evaluated Using Advanced Diffusion MRI Biomarkers
Date: 08/2023
Authors: Maurizio Bergamino, Sana Aslam, Jacob J. Knittel, Lea Alhilali, Lea M. Alhilali, Ashley M. Stokes
Identification of single-dose, dual-echo based CBV threshold for fractional tumor burden mapping in recurrent glioblastoma
Date: 01/2023
Authors: Aliya Anil, Ashley M. Stokes, Renee Chao, Leland S. Hu, Lea Alhilali, Lea M Alhilali, John P. Karis, J. P. Karis, John P Karis, Laura C. Bell, C Chad Quarles
Structural connectivity and brain network analyses in Parkinson’s disease: A cross-sectional and longitudinal study
Date: 01/2023
Authors: Maurizio Bergamino, Elizabeth G. Keeling, Nicola J. Ray, Antonella Macerollo, Monty Silverdale, Ashley M. Stokes
Principal Investigator
Investigators

Postdoctoral Fellows

Graduate Students

Research Coordinators







